Gut microbiome and neurosurgery: Implications for treatment
[Correction added on November 5, 2022 after first online publication: the Author Contributions, Acknowledgments, Funding, Conflicts of Interest, Data Availability Statement and Ethical approval has been updated].
Abstract
Introduction
The aim of this review is to summarize the current understanding of the gut-brain axis (GBA), its impact on neurosurgery, and its implications for future treatment.
Background
An abundance of research has established the existence of a collection of pathways between the gut microbiome and the central nervous system (CNS), commonly known as the GBA. Complicating this relationship, the gut microbiome bacterial diversity appears to change with age, antibiotic exposure and a number of external and internal factors.
Methods
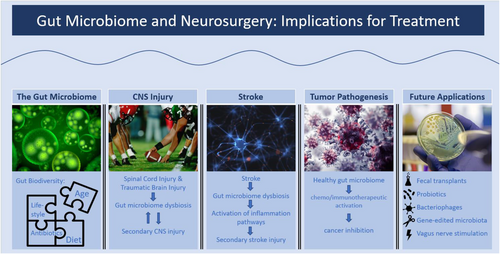
In this paper, we present the current understanding of the key protective and deleterious roles the gut microbiome plays in the pathogenesis of several common neurosurgical concerns.
Results
Specifically, we examine how spinal cord injury, traumatic brain injury and stroke may cause gut microbial dysbiosis. Furthermore, this link appears to be bidirectional as gut dysbiosis contributes to secondary CNS injury in each of these ailment settings. This toxic cycle may be broken, and the future secondary damage rescued by timely, therapeutic, gut microbiome modification. In addition, a robust gut microbiome appears to improve outcomes in brain tumour treatment. There are several primary routes by which microbiome dysbiosis may be ameliorated, including faecal microbiota transplant, oral probiotics, bacteriophages, genetic modification of gut microbiota and vagus nerve stimulation.
Conclusion
The GBA represents an important component of patient care in the field of neurosurgery. Future research may illuminate ideal methods of therapeutic microbiome modulation in distinct pathogenic settings.
1 INTRODUCTION
Microbiome dysregulation has been implicated in the pathogenesis and prognosis of a wide range of conditions, from type 2 diabetes to Parkinson's disease.1-3 Recent research in the field of microbiome study has shown a particular interest in the gut-brain axis (GBA), as numerous studies have suggested that gut microbiota modulate neuroendocrine signalling pathways via the production of neurotransmitters (e.g. serotonin, norepinephrine, dopamine, glutamate and gamma-aminobutyric acid) and metabolites (e.g. tryptophan).4-6 Although the direct relationship between the gut and the brain is not obvious, research has established evidence on the GBA bidirectional communication via different systems.7 For instance, the gut microbiome is known to communicate to the central nervous system (CNS) via the myenteric systems and the entero-endocrine cells using various neuropeptides (5-Hydroxytryptamine, Peptide YY and Cholecystokinin) and directly influence behavioural changes and cerebral cortical excitability.8 On the other hand, the CNS, using the parasympathetic fibres from the vagus nerve (VN), directly influences the composition of the gut microbiome by either activating anti-inflammatory macrophages (M2) or inhibiting proinflammatory macrophages (M1), hence modulating intestinal permeability.9
The diversity of the microbiota in early life is influenced by a variety of factors, including the route of birth delivery, breast milk consumption, genetics, nutrition, infection history, antibiotic history and environmental stressors.10-13 As the host ages, microbiota diversity is believed to generally trend towards a less diverse microbiome, with potential consequences of increased inflammation.14, 15
In either acute or chronic settings, dysbiosis describes an overall decrease in microbiota diversity with an inability to regulate pathogenic gut microbes, promoting a proinflammatory state.16, 17 The bidirectional GBA impact of CNS injury and malignancy resulting in gut microbiome dysregulation and increased intestinal permeability may further drive systemic inflammation and secondary CNS injury.18-20 Dysbiosis in the setting of neural injuries such as strokes and traumatic brain injuries (TBIs) has been indicated in the differentiation and migration of immune cells into the CNS, and ultimately the upregulation of maladaptive CNS inflammation.19, 21 Depending on the predominate microbiota, the GBA may promote an inflammatory or anti-inflammatory reaction via the upregulation of specific cytokines and antioxidants.22 Spinal cord injury (SCI), TBI, stroke and brain tumours appear to have a significant impact on the GBA, influencing dysbiosis with secondary consequences on CNS dysregulation.22-25
2 THE GUT MICROBIOME
The human gut microbiome is home to many species, including bacteria, viruses, fungi, protozoa and some eukaryotes, more so than any other anatomical region.26, 27 These microorganisms colonize the digestive tract just after birth and have been implicated in several significant functions in the human body, ranging from enhancing the immune system, colonizing mucosal surfaces, playing vital roles in digestion and metabolism, modifying insulin resistance and influencing the GBA, which influences the neurological function of the host.28-31 Consequently, there is an abundance of evidence supporting the concept that gut microbiota plays a crucial role in maintaining normal gut physiology and health within several body systems.
2.1 Microbiome diversity
The composition of the gut microbiome varies across different areas of the digestive tract, but the majority of the microorganisms are bacteria, specifically anaerobic bacteria.32 The total microbiome also includes fungi, protists, archaea and viruses, but much less is known about their specific roles.33 The colon is the most densely populated area of the digestive tract, with 1012 cells per gram of intestinal substance comprised of up to 1000 different species.33, 34 However, a majority (99%) of those bacterial species originate from a central group of about 40 species.34 While most of the digestive tract is colonized by anaerobic bacteria, there is a high concentration of aerobic bacteria in the cecum.33 The most common bacterial genera include Bacteroides, Clostridium, Peptococcus, Bifidobacterium, Eubacterium, Ruminococcus, Faecalibacterium and Peptostreptococcus, with Bacteroides being the most prevalent as it comprises about 30% of gut bacteria.34, 35 Bacterial categorization is commonly established by phyla, as the ratio of certain phylae has significant implications for research results and the conclusions that can be drawn regarding gut health. More than 50 different phyla have been recorded, with Bacteroidetes and Firmicutes followed by Proteobacteria, Fusobacteria, Tenericutes, Actinobacteria and Verrucomicrobia being the most common.36, 37
2.2 Age and the gut microbiome composition
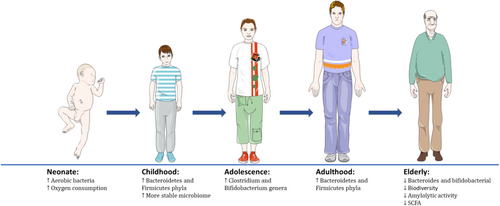
The gut microbiome changes composition throughout life, as it is affected by several factors, including host genetics, age, diet, mode of birth, exercise, lifestyle and antibiotic use.38-40 In healthy adults, there are generally high quantities of Firmicutes and Bacteroidetes and also lesser proportions of Verrucomicrobia, Actinobacteria and Proteobacteria.41 However, this composition does not remain constant as natural ageing significantly affects the microbiota's composition (see Figure 1).33 Initially, aerobic bacterial strains (e.g. Proteobacteria) colonize the gut and consume oxygen, which sets up the optimal environment for anaerobic strains to subsequently colonize (examples include Firmicutes, Actinobacteria and Bacteroides).42 The first year after birth is quite crucial in establishing the optimal microbiome, as taxonomic diversity initially is low, but develops over time.43 During childhood, the more common phyla, such as Bacteroidetes and Firmicutes, begin to multiply, leading to a more stable microbiome that can produce butyrates.44 The pre-adolescent years (7–12 years old) then allow for folate and vitamin B12 synthesis.45 The primary difference during adolescent years (11–18 years old) is the increasing abundance of Clostridium and Bifidobacterium genera, at higher levels compared to those of healthy adults.46 As humans age, the Bacteroidetes:Firmicutes ratio lowers, as Enterobacteriaceae quantities seem to increase.47 Although there is no consensus on the reason for this change, prevalent hypotheses include a decrease in diet diversity and an increase in inflammatory factors.48 Furthermore, along with a significant reduction in bifidobacteria levels, there is also a decrease in amylolytic activity and short-chain fatty acid (SCFA) production, due to the reduction of Bacteroides levels.33, 49 There is a corresponding increase in the number of facultative anaerobes, fusobacteria, clostridia and eubacteria.49 Ultimately, due to the decrease in biodiversity and SCFA-producing species, clinical manifestations include more opportunistic pathogen infections, a decline in dentition, salivary function and digestion/absorption function overall.50
2.3 Diet and the gut microbiome composition
In addition to natural ageing, a human's dietary intake plays a crucial role in regulating the gut microbiome, by increasing or decreasing quantities of certain bacterial species, which may alter gut metabolite production.51 For example, shortly after birth, the microbiome's metagenome is enriched in genes that metabolize oligosaccharides found in breast milk.52 With time, this transforms into an upregulation of genes suited for the metabolization of polysaccharides and vitamins.52 Furthermore, the method of feeding has been shown to strongly influence the microbiome composition, with breastfeeding leading to a high abundance of Actinobacteria and an inhibition of Firmicutes and Proteobacteria, while formula leads to an abundance of Clostridia, Streptococci, Bacteroides and Enterobacteria.33, 53, 54 Certain adult diets have also been associated with certain microbiome compositions. For instance, vegetarian diets are correlated with the dominance of Firmicutes and Bacteroidetes.33 In addition, increased fibre intake has been shown to protect the gut mucosal barrier and improve glucose control, leading to a healthier metabolic profile in T2DM patients.55, 56 On the other hand, diets rich in protein and fats (most associated with diets consumed in European countries), are correlated with Bacteroides, Bilophila and Alistipes (bile-tolerant species) and inhibition of Firmicutes.57, 58 Predominance of this diet type is also associated with lowered immune function (and hence, susceptibility to infection and metabolic diseases).37
2.4 Lifestyle and the gut microbiome composition
Another major factor believed to modulate microbiome composition is a lifestyle, which encompasses a plethora of factors from host genetics to exercise to habits such as smoking.33 Exercise appears to enhance the diversity of the microbiome and is positively correlated with protein intake and creatine kinase levels.59 Athletes display greater quantities of Firmicutes and lower levels of Bacteroidetes in relation to non-athletes.33 In general, exercise seems to increase certain taxa (e.g. Clostridiales and Roseburia) that increase butyrate production and SCFA production (a general indicator of gut health).60
While their exact mechanisms remain uncertain, both intrinsic and extrinsic factors clearly play a role in microbiome composition. Intrinsic adaptations include decreased blood flow and tissue hypoxia while extrinsic factors include the general environmental biosphere. Smoking is one example of an extrinsic factor which has been shown to significantly influence gut microbiota.61
2.5 Inflammation and the gut microbiome
While several phyla mentioned thus far are said to be beneficial for physiological health, there are known bacteria with pro-inflammatory properties, which have been correlated with several diseases, including metabolic syndrome, inflammatory bowel disease (IBD), obesity and more.62 Enterobacteriaceae is one such class of gram-negative facultative bacteria that is believed to contribute to an inflammatory state.62, 63 In general, bacteria mediate pro-inflammatory effects by acting on macrophages (differentiating between M1 and M2 phenotypes) through the production of Gram-negative bacterial lipopolysaccharides (LPS), bile acids, and pro-inflammatory cytokines (e.g. IL-8, IL-12, IL-18, IL-23, TNF-α and IL-1β).62, 64
2.6 The GBA
Another significant relationship between the microbiome and human health is through the GBA, whereby the enteric and CNS are bidirectionally linked.51, 65 The GBA's primary function is to connect the emotional centres of the brain with the peripheral intestinal functions such as the enteric reflex, intestinal permeability, immune activation and enteroendocrine signalling.66, 67 A plethora of evidence point to this connection, and dysfunction of the GBA has been implicated in several neurological and psychiatric diseases (e.g. anxiety, depression, autism and Alzheimer's disease).68 The GBA is disrupted in IBD and microbial dysbiosis.69 In these pathological states, intestinal motility and secretion are disrupted, leading to visceral hypersensitivity or hyperalgesia.70
Additionally, a leading hypothesis is that a “leaky” gut allows for metabolites and pro-inflammatory molecules to leak into the bloodstream, which can then alter neuroimmune and neuroendocrine systems, leading to the pathogenesis of autism and other alterations of physiologic neurodevelopment.71, 72
To further explore the GBA, neurodevelopment has been studied extensively in mice, as mice share two major phyla of bacteria with humans (Bacteroidetes and Firmicutes) and are one of the best living GBA models, despite several differences in microbiome composition amongst mice and humans.73 Specifically, germ-free (GF) mice, those devoid of any microbiota, have been used to study the impact of an absent microbiome and the host's physiological response.74 Typical experimental groups can compare GF mice, conventionally raised mice and mice raised devoid of specific disease-causing organisms.75 One study comparing GF and conventionally raised mice indicated a number of behavioural changes, including stress and anxiety-related responses, affected learning, memory and motor control skills, which seemed to parallel behaviour seen in autism spectrum disorder.76 Another study showed a significant decrease in neuronal firing rate and increase in synaptic density in GF neonate mice when compared to conventional neonate mice.77 This substantial role of bacteria in neuronal development was further supported by Lu et al. who demonstrated that GF mice colonized by faecal samples from preterm human infants with poor growth compared to GF mice colonized by faecal samples from infants with good growth showed a significant reduction in neuronal markers, neurofilament-L, and myelination markers.78
Other studies have explored how the integrity of the blood-brain barrier (BBB) could be affected by the GBA. It was found that increased levels of circulating SCFAs produced by the gut microbiome led to increased production of tight junction proteins, causing increased BBB integrity, and preventing entry of unwanted metabolites into brain tissue.79 Moreover, the effect of pro-inflammatory bacteria via altered autoimmune function was also supported via GF mice. More specifically, there is evidence that LPS stimulate cytokine release, which then cross the BBB, and alter neurological function, resulting in modulated mood and behaviour.80 Ultimately, GF mice have allowed for further study of the correlation between gut microbiome alterations and neurodevelopment. However, it is important to note the limitations given that a GF environment is not plausible in real life, limiting the transitional value of such studies to human therapeutics or reaching clinically relevant conclusions.75
3 SPINAL CORD INJURY
SCI affects approximately 250 000–500 000 people per year worldwide and is associated with significant morbidity and mortality.81 Emerging evidence has demonstrated a bidirectional effect of SCI and the microbiome as SCI can result in gut dysbiosis which can negatively impact recovery following SCI (see Figure 2). Thus, understanding the interaction between the gut microbiome and SCI is necessary, as preventing gut dysbiosis could become an important component of post-SCI care. SCI is a combined upper and lower motor neuron CNS injury that can result in dysfunctional somatic and autonomic nervous systems.82-84 Lesions of upper and lower motor neurons in these systems which, along with the enteric nervous system, control gastrointestinal (GI) function can result in neurogenic bowel and reduced stool transit time, colon motility, mucosal secretions, vascular tone, gastric dilation, intestinal barrier integrity and immune functioning.85, 86 This can, in turn, lead to microbiome dysbiosis.85, 86 Additionally, the gastrointestinal-associated lymphoid tissue (GALT), which prevents the gut microbes from invading GI mucosa, is normally innervated and suppressed by the sympathetic nervous system. Consequently, loss of sympathetic tone in the setting of SCI increases the antimicrobial function of GALT, further contributing to gut dysbiosis.87
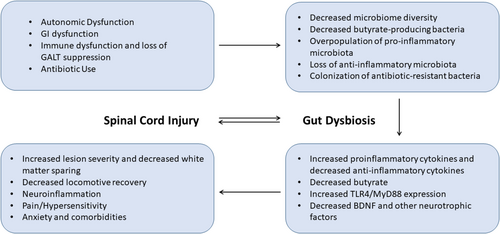
SCI patients suffer from defective immune system function, resulting in recurrent infections requiring frequent use of antibiotics, which leads to antimicrobial resistance of some colonized bacteria in the GI tract and alterations in the populations of microbes present in the gut.85, 87-91 After SCI, there is a loss of diversity in the gut microbiome.92-98 The changes in specific microbiota populations are variable in preclinical rodent studies due to differences in injury type, severity and location as well as post-op care protocols.99-102 Generally, there is a decrease in anti-inflammatory bacteria and an increase in pro-inflammatory, potentially pathogenic microbiota.92, 93, 103-109 In human studies, changes in bacteria are also variable between individuals due to variations in injury levels and severity as well as antibiotic usage, age, diet, lifestyle, prolonged stress and GI dysfunction.98, 17 However, human studies have demonstrated a reduction in butyrate-producing microbiota after SCI.84, 98
3.1 Gut dysbiosis and secondary SCI perturbation
Gut microbiome dysbiosis can negatively impact injury severity and inflammation. Mice with SCI and gut dysbiosis have more severe lesions104, 109, 110 with less white matter sparing.104, 110 Mice with SCI treated with faecal microbiota transplantation (FMT) from an uninjured mouse have a significant increase in motor neurons relative to mice with SCI without FMT, suggesting that cell survival is improved by ameliorating microbiome dysbiosis.93, 110 Gut dysbiosis may result in a more severe secondary injury due to a more pronounced proinflammatory state, thus worsening lesion severity and reducing white matter sparing. Inflammation is a key component of the secondary injury in SCI and is mediated through pro-inflammatory cytokines and pathways, microgliosis, astrogliosis, and infiltrating immune cells.111-114 Mice with SCI and gut dysbiosis have increased peak inflammation at 14 days.104 Spinal cord injury results in intestinal permeability changes that allow for bacteria in the gut and their metabolites to invade the gut mucosa and enter circulation, resulting in systemic inflammation.86, 111 Additionally, loss of sympathetic innervation of GALT results in changes in immune cell composition which results in increased expression of pro-inflammatory and immunoregulatory cytokines.104, 111 Rong et al. found that at 7 days post-injury, mice with SCI and gut dysbiosis have increased proinflammatory cytokines such as TNF-α, IL-1β and IL6 and decreased anti-inflammatory cytokines such as TGF-β, IL-4 and IL-10.109 Jing et al. found that 4 weeks post-injury, mice with SCI have elevated NFκB and IL-1β which can be impeded through treatment with FMT from a sham mouse. O'Connor et al. found that elevations in pro-inflammatory cytokines IL-1β, IL-12 and MIP-2 correlate with microbiome changes following SCI.92 Mice with gut dysbiosis after SCI have increased immunofluorescent staining for GFAP and IBA-1 as well as increased astrocytes and microglia with activated morphology, indicating increased astrogliosis and microgliosis.104, 109, 110 Treatment with FMT from a sham mouse reduces the activated morphology of astrocytes and microglia indicating that ameliorating gut dysbiosis in SCI prevents the overactivation of glia.110 While glia are important in the response to SCI, there is a delicate balance between pro- and anti-inflammatory states, which if unbalanced can worsen the secondary injury.111, 115, 116 Microglia in the M1 state are pro-inflammatory and can further contribute to secondary injury.111, 116 The reduction of butyrate-producing microbes seen after SCI can result in more microglia with the M1 phenotype.117 M1 microglia release proinflammatory cytokines and recruit immune cells to the injury, altogether leading to inflammation, cell death, demyelination, nociceptive activation, and hypersensitivity.117 The mice with both SCI and dysbiosis have an increased total number of infiltrating lymphocytes.104 These mice also have increased expression of the spinal cord and colon TLR4, MyD88, p-p65 and p-IκBα, important molecules in the inflammatory pathway that responds to LPS.109
3.2 Neuronal recovery and gut dysbiosis
Gut dysbiosis in SCI results in worsened locomotive recovery.93, 104 Gut dysbiosis may dampen the potential for neuroplasticity after SCI, thus reducing the potential for functional recovery. The mechanisms for neural plasticity after SCI and how to enhance that plasticity to improve rehabilitation are still being elucidated. The increased inflammation may blunt the potential for neuroplasticity.118-121 Gut dysbiosis post-SCF could also impact important molecules involved in neuroplasticity after SCI, such as BDNF and other neurotrophic factors.122-126 The intestinal microbiome modulates BDNF messenger RNA affecting the capacity for long-term potentiation in the hippocampus.127-129 Additionally, inflammatory cytokines such as IL-1β, which is overexpressed in SCI, especially with gut dysbiosis, can suppress BDNF-dependent pathways of synaptic plasticity.110, 120, 130 Mice with SCI have a significant decrease in expression of BDNF, NT-3 and NGF in the spinal cord, 4 weeks post-injury.110 Mice with SCI treated with FMT from a sham mouse, have BDNF, NT-3 and NGF expression levels that are significantly improved relative to that of SCI mice without FMT and are not significantly different from that of non-SCI mice.110 The gut microbiome also mediates the biosynthesis of tryptophan, vitamin B6 and folate which are important to CNS repair and neuroplasticity.86, 103 Tryptophan and vitamin B6 are important in the synthesis of serotonin,103 and the gut is responsible for a large amount of the serotonin supply in the CNS.131 Serotonin is another molecule implicated in a pathway important for neuroplasticity after SCI.119, 132-136 It is not yet known how the microbiome perturbation post-SCI affects serotonin or serotonin-mediated neuroplasticity pathways. Future research should be performed to determine how SCI and the resulting gut dysbiosis modulate key players in neuroplasticity pathways such as serotonin, adenosine, BDNF and mTOR and how those changes impact the capacity for plasticity. In addition to worsened inflammation and recovery, gut dysbiosis post-SCI can also contribute to the development of comorbidities such as stress and mood disorders, immune dysfunction and infections, metabolic disease and fatigue.85, 87, 94, 107, 108
3.3 FMT in the setting of SCI
As modern research continues to highlight the importance of the microbiome in SCI prognosis, it is clear that gut dysbiosis should be considered a target for post-SCI care.
Probiotics work by reintroducing specific, beneficial bacteria to the GI tract.104, 137 VSL#3 is a commercial, medical-grade probiotic cocktail that contains four strains of Lactobacillus (L.casei, L. plantarum, L. acidophilus and L. delbrueckii), three strains of Bifidobacterium (B. longum, B. breve and B. infantis) and one strain of Streptococcus (S. salivarius).104, 137, 138 In a mouse study, VSL#3 transiently but significantly altered the microbiome composition in faecal samples, and notably increased the abundance of Lactobacillus and Bifidobacterium.104 Mice treated with VSL#3 starting at the time of SCI and continued for 35 days have significantly improved locomotor recovery, increased activation of anti-inflammatory T-regulatory cells, and reduced lesion volume and axon/myelin pathology as compared to SCI mice without probiotic treatment.104 FMT involves transferring faeces or faecal microbiota from the GI tract of a healthy donor to the GI tract of the recipient. In mice studies of FMT after SCI, FMT treated mice had less severe injuries with more white matter sparing, greater locomotive recovery, enhanced vascular repair, upregulated neurotrophic factors, reduced neuroinflammation and decreased blood-spinal cord barrier disruption as compared to untreated SCI mice.93, 110 Melatonin is another potential therapeutic in addressing gut dysbiosis in SCI. Mice treated with melatonin after SCI have a more normalized gut microbiome and significantly improved GI function.106 The correction of microbiota disturbances in the mice treated with melatonin correlated with improved functional recovery.106 Ursolic acid has also been shown to improve the gut microbiome diversity and composition following SCI, decrease the pro-inflammatory response, and promote cell survival.139 It is likely that melatonin and ursolic acid work through mechanisms, both related and unrelated to the microbiome.106, 139 Future work should aim to uncover the optimal treatment strategy for addressing gut dysbiosis in SCI and preventing the gut microbiome's contribution to increased secondary injury and limited plasticity and recovery.
4 TRAUMATIC BRAIN INJURY
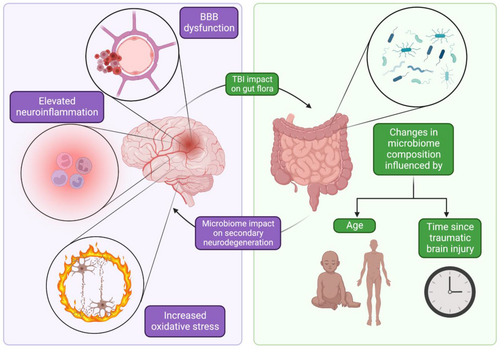
TBI is a significant public health issue, accounting for an estimated third of deaths related to trauma or injury.140 Recently, the gut microbiome has been a focus of interest in the context of TBI following a number of findings supporting the codependent influence of brain injury on the health of the microbiome and vice versa. Here, we describe these findings (see Figure 3) and interweave a discussion of the modulation of the gut microbiome as a therapeutic target to reduce secondary neurodegeneration following TBI.
4.1 The gut microbiome impacts recovery after TBI
It is becoming increasingly evident that the gut microbiome plays a key role in the regulation of neural recovery following TBI. One recent study investigated the effect of gut dysbiosis on recovery with an antibiotic-treated mouse model subjected to an impact model of TBI.141 Compared to control animals, antibiotic-treated mice displayed increased hippocampal neurodegeneration and altered cognition in the form of a fear memory freezing task following TBI.141 Despite this, the underlying mechanisms to which these dysbiotic neurodegenerative changes may be attributed are not fully understood. However, three possible mechanisms include microbiome-induced regulation of BBB integrity, neuroinflammation and oxidative stress. BBB dysfunction with resultant increased permeability is a well-known sequela of TBI.142 Further, the gut microbiome is known to regulate the permeability of the BBB: GF mice have decreased expression of tight junction proteins (with high BBB permeability) and faecal transplant of healthy gut flora to these mice results in reduced BBB permeability.143 These findings were supported in a study of TBI impact model mice, where intragastric Clostridium butyricum (Cb) treated mice not only rescued BBB permeability but also improved neurological deficits compared to the control group not treated with Cb.144 Neuroinflammation contributes to degeneration following TBI and is also influenced by gut flora.145, 18 Cb treatment has also been shown to reduce the expression of proinflammatory cytokines such as IL-6.145 Furthermore, elevated oxidative stress in the neural environment plays a significant role in inducing apoptosis after TBI.146 The gut microbiome has also been found to regulate this mechanism of neurodegeneration. In one study, rats were subjected to an impact model of TBI, and a faecal transplant was performed for one week following injury.147 Fecal transplant was associated with reduced ROS and elevated antioxidant enzymes such as superoxide dismutase and catalase, corroborated with improved neurological function following transplant.147
The mechanism of TBI-induced GBA dysfunction is not fully understood, but the VN is one proposed major component. Recent studies suggest the bidirectional nature of the VN allows it to function as a major pathway of communication between the GBA.148, 149 Consequently, the VN may play an integral role in the promotion of decreased gut mucosal integrity, inflammatory upregulation, and apoptosis in the setting of TBI-induced dysbiosis.
4.2 The influence of TBI on the microbiome
Just as the state of the microbiome influences neurological outcomes in TBI, neurological injury can have several effects on the microbiome itself. One study comparing mice subjected to an impact model of TBI to sham mice analyzed the stool microbiome 24 hours following injury. This study reported changes in the microbiome composition in the TBI-induced mice, attesting to the acute changes in the microbiome following TBI.150, 151 These changes may not be limited to the immediate post-injury period: TBI could have long-lasting effects on the gut flora profile, as discovered in a study comparing the microbiome composition of 22 patients who suffered moderate-to-severe TBI with 18 age-matched controls.152 Nevertheless, controversy regarding the long-term implications of TBI on the gut microbiome exists and warrants further research.153 Though this study compared microbiome profiles to age-matched controls, other recent work has implicated age difference as a critical influence on the post-TBI gut microenvironment. Notably, older mice subjected to TBI displayed different gut flora environments than younger mice.154 Indeed, the microbiome may undergo a spectrum of changes over time following TBI, making analysis of gut flora a possible venue for injury prognostication.155 However, TBI-induced changes in the gut microbiome may be alleviated through faecal transplant which, again, has been demonstrated to improve cognitive performance in mice.156
5 BRAIN TUMOR PATHOGENESIS
Many studies report an existing relationship between the gut microbiome and human cancer development, including brain tumours.157-159 Animal models demonstrated that gut microbiome-driven changes to microglia in the CNS have a direct impact on CNS disease.160 Such changes are either direct or indirect. For instance, GF mice seem to have a higher propensity of having microglia abnormalities leading to CNS disease and cancer development.161 Interestingly, when treated with faecal implantation, such effects are hampered.160 It appears that secreted specific SCFA from gut microbes act as neurotransmitters to act on the CNS microglia.162 Gliomas account for 80% of all brain cancers and are one of the most aggressive.163 It is reported that gut microbiomes directly affect their growth and their response to therapy164; mostly, either by leading to an increase in NK cell subtypes (CD27+/CD11b+)161 or by modulating oncogenes.165, 166
5.1 The GBA
Cancer therapy has come to know a new era with the use of immunotherapy and is being met with tremendous success.167 Multiple factors have been demonstrated to significantly influence the results of such therapy ranging from modifiable ones, such as lifestyle, to non-modifiable ones, such as genetic inheritance.159 Recently, the implication of the gut microbiome in cancer response to therapy became an area of research interest in the field of immuno-oncology and is being investigated as a possible tool to enhance therapeutic efficacy.168-170
5.2 The gut microbiome and efficacy of chemotherapeutics and immunotherapeutics
Unfortunately, the direct action of chemotherapeutic drugs leads to significant gastrointestinal side effects by affecting the microbiome diversity.170, 171 Therefore, there is a decrease in the bioavailability of those drugs. Regulating the gut microbiome by providing external probiotics can hamper those toxicities and improve chemotherapeutic agent efficacy.170 Furthermore, genetically altered bacteria species can help in drug metabolism and even secrete anti-tumour agents that benefit the function of chemotherapy.172, 173 Animal and human models have also demonstrated how the gut microbiome affects the response to cancer immunotherapy. Although the mechanism is not completely understood, Cytotoxic T Lymphocytes Associated Antigen 4 and PD-1 targeted therapy success depends greatly on the host's gut microbiome.174 It is believed that if these areas are further investigated, understanding the gut microbiome can greatly assist in prognostication and guide therapeutic intervention against cancer.175
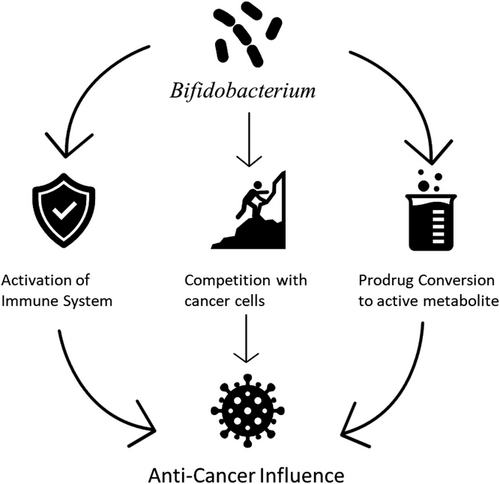
Programmed Death 1 (PD-1) is a transmembrane protein in the family of immunoglobulins found mostly on lymphocytes that play a role in immunoregulation. PD-1 has two main ligands, PDL1 and PDL2, to which naturally occurring, or biologically engineered molecules can attach.176 When molecules are attached to the ligands, CD4+ naive cells are transformed into regulatory T-cells177 and subsequently lead to the induction of Akt-mammalian target of rapamycin (mTOR) and activation of Phosphatase and Tensin homolog (PTEN).178, 179 Such events are immunosuppressive and, therefore, decrease the ability of the organism to counteract pro-inflammatory states and malignancies. This explains why various tumour-infiltrating lymphocytes have a higher rate of recurrence and poor prognosis.180-182 Some molecules, however, act as PD-1 inhibitors. Naturally occurring molecules include Anoectochilus formonasus, Curcumin and Resveratrol.183 Studies have also demonstrated that in certain patients, the presence of some species of bacteria in the patients’ microbiome confers primary anti-tumour immunity and were also found to improve the efficacy of the immune checkpoint inhibitors utilized in cancer treatment. Species like Clostridium, Lactobacillus and Syntrophococcus as well as bacteria like Alistipes putredinis, Bifidobacterium longum and Prevotella copri have been cited.184 Particular attention was given to Bifidobacterium spp. after Sivan et al. elegantly demonstrated that its presence in certain melanoma-affected mice contributes to spontaneous anti-tumour immunity and synergistically works with immune checkpoint inhibitors to decrease disease burden, while its absence correlates with the lack of such responses.185 The mechanisms by which Bifidobacterium spp. produces these effects are not well elucidated. However, the direct correlation between Bifidobacterium spp. presence and CD8+ concentration within the tumour and in the host's circulation as well as the increased production of IFN-γ suggest gene-expression-related activities.165, 166 This is postulated to be the results of yet-to-be-determined signalling, as there was no observed evidence of bacterial translocation. Other proposed mechanisms include biotransformation assisting in prodrug conversion into active metabolites186 and competition with cancer cells, reducing the nutrients available for cancer growth (see Figure 4).187
6 STROKE
A stroke is defined as brain tissue injury resulting from inadequate blood flow due to either vascular occlusions and stenosis in about 85% of cases or vascular rupture in 15% of cases.188-191 Recent evidence suggests that the bidirectional GBA may play a significant role in stroke pathophysiology and post-stroke secondary injury.192, 193 Since the gut microbiome is also responsible for regulating T-cell homeostasis and immune cell maturation, alterations in the composition and abundance of microbes resulting in gut dysbiosis may disrupt GBA signalling and GBA-mediated immune responses.194-197
6.1 Stroke-related gut dysbiosis
Stroke-related gut dysbiosis is well documented in the literature. Compared to healthy controls and those with gastrointestinal conditions such as ulcerative colitis, stroke patients have been shown to have an: 1) altered Firmicutes-to-Bacteroidetes ratio, 2) increased abundance of opportunistic pathogens and 3) decreased abundance of SCFA-producing bacteria.198-202 The degree of stroke-related gut dysbiosis has also been shown to be strongly and positively associated with stroke severity based on the National Institute of Health Stroke Score and post-stroke disability based on the Modified Rankin Scale.199 Those with poor post-stroke outcomes were found to have the same three major changes in gut microbiome composition but to a greater extent.203 Understanding this phenomenon is important, as gut dysbiosis has been associated with diminished survival. Spychala et al. used stroke mice models to show that aged mice or those with aged gut microbiota had an elevated Firmicutes to Bacteroidetes (F:B) ratio which was associated with lessened survival and post-stroke recovery.204
6.2 Proinflammatory effect of stroke-related gut dysbiosis
Gut dysbiosis and its role in the progression of inflammatory events post-stroke have been well studied (see figure 5). Xu et al. showed in a middle cerebral artery occlusion (MCAO), a murine model that brain infarction size was associated with gut Enterobacteriaceae overgrowth, and through RNA sequencing found these microbial species to have upregulated genes involved in immune responses.205 Specifically, they showed increased interferon, NOD-like receptor and toll-like receptor signalling. Further serum testing also demonstrated increased systemic inflammatory markers including IL-6, LPS and TNF-α.

Post-stroke systemic inflammation is believed to be driven through various mechanisms, with a major source being stroke-related changes in intestinal mucosal permeability. Multiple studies have shown decreased expression of occludins, claudins and cadherins within intestinal mucosa following stroke.206-209 Without proper cell-to-cell adhesion and tight junctions, mucosal integrity is compromised. MicroRNA has also been shown to be involved in changes in intestinal permeability. MiR-21-5p is one specific microRNA that has been shown to be elevated in stroke patient serum and is associated with increased intestinal epithelial permeability via upregulation of the ADP-ribosylation factor.210, 211 The resultant “leaky gut” allows opportunistic pathogens that bloom as a result of gut dysbiosis to produce and release harmful metabolites and toxins into the systemic circulation. Kurita et al. showed in an MCAO murine model that infarct volumes were associated with both gut dysbiosis—especially increased Gram-negative Enterobacteriaceae—and increased lipopolysaccharide, an inflammatory stimulus and endotoxin, near the ischemic insult.212 Accordingly, Stanley et al. have shown in humans and animals, that microbes detected in post-stroke infections were innate to the gastrointestinal tract, and that microbe translocation occurred after stroke-related changes in intestinal permeability.213
Ultimately, the proinflammatory pathogenic microbes in the gut and subsequent activation of the gut immune cells drive systemic inflammation and contribute to inflammation at the site of ischemia. This is due to the mechanism by which brain tissue injury following ischemia also results in the localized release of damage-associated molecular patterns and proinflammatory cytokines that can attract gut immune cells.192 This was studied by Singh et al. who fluorescently labelled immune cells in Peyer's patches and tracked these cells 3 days later to the ischemic hemisphere in MCAO mice models.195 Interestingly, these cells - mostly consisting of TH1 and TH17 cells - accounted for 25% of the total T cells at the site of ischemia. Additionally, proinflammatory factors released from gut immune cells can disrupt the BBB and allow gut-lumen-derived pathogens to reach the brain and trigger further neuroinflammation.214 Certain endotoxins released by opportunistic microbes such as LPS have even been associated with microgliosis, neurotoxicity and increased cognitive impairment.215
7 THE FUTURE OF MICROBIOME THERAPEUTICS
Treating neurological pathology via directed modulation of gastrointestinal microbiota is an ongoing field of research. Current and theorized interventions may be grossly categorized into the transferring of wild-type gut microbes, such as with FMT and Probiotics216; the elimination of endogenous gut microbes via virulent bacteriophages217; and either the introduction of exogenously engineered microbes or the genetic modification of endogenous ones.218 Research has also demonstrated the potential for individualized gut microbiota diagnostics using engineered bacteria, which could lead to personalized microbiota-targeted therapies.219 However, further research is required to fully implement these methods in the clinical setting.
7.1 Faecal microbiota transplant
FMT is the transplantation of faecal material from a donor with apparent healthy gastrointestinal flora into a diseased recipient to correct dysbiosis.220, 221 Records of FMT date back as far as 4th-century China, where it was used to treat severe diarrhoea.222 In the modern era, FMT has been explored as a highly effective treatment for recurrent Clostridium difficilie infection (CID).223 Other potential indications for FMT have been studied in clinical trials, but have yielded heterogeneous results.224, 225 However, in the setting of induced TBI in rat-based models, FMT has demonstrated efficacy in the amelioration of both gut microbiota dysbiosis and neurological deficits.226, 93 The exact therapeutic mechanism by which FMT treats CID is still not fully realized, but the most current evidence points towards its ability to repopulate and revitalize the diseased gut with diverse and healthy microbiota.227, 228
7.2 Probiotics
Probiotic is a general term used when referring to specific health-promoting microbes, commonly found in certain foods, that have demonstrated some beneficial effect on human health.229 Probiotics as a food supplement are largely considered safe and have been shown to improve intestinal health by modulating the immune system, producing organic acids and improving gut barrier function. The specific means by which probiotics exert these effects are still being investigated, but it is thought to function similarly to FMT by introducing healthy microbiota.230 Both Du et al. and Yi et al., in two independent meta-analyses comprising 39 and 18 clinical trials, respectively, found that probiotics supplementation in enteral nutrition resulted in a decreased risk of mortality, infection and gastrointestinal complications in patients suffering from a severe head injury.231, 232
7.3 Bacteriophages
Bacteriophages (commonly referred to as “phages”) are a class of prokaryotic viruses that have evolved specifically to infect and replicate within bacteria.233 These viruses are naturally found within the gut, and there is evidence to suggest that the gut phageome is individual-specific and plays an active role in healthy gut function similar to bacteria.234, 235 One study even showed that gut phages may play a causal role in FMT's treatment of CDI236 As such, there is a growing bodying of research investigating the use of virulent phages in gut microbiota-related diseases through the direct elimination of targeted communal strains. Duan et. al. successfully treated alcohol-related liver disease in mice via directed targeting and subsequently reducing populations of cytolysin-producing E. faecalis with a cocktail of virulent phages. Further research in human trials would be required to validate these results, but this study lends support to using phages in the treatment of other conditions by directly reducing the population of specific bacteria within the gut microbiome.217 Additionally, a randomized controlled trial is currently underway studying the use of engineered phages to treat catheter-associated UTIs in patients with SCI.237, 238
7.4 Genetic modification of gut microbiota
Treating disease through the use of genetically engineered bacteria is an active field of research, indicating promising results for conditions such as type I diabetes, phenylketonuria, hyperammonemia, fungal infection and colorectal cancer.239-241 Therapeutic interventions of genetically modified bacteria vary, but methods may be grossly organized into either engineering bacteria in vitro and introducing them into the host exogenously,242 or genetically modifying endogenous gut microbiota in vivo.243 In addition to directly modifying gut microbiota, genetically engineered bacteria have also been studied for use as a non-invasive in vivo diagnostic modality for current gut health. Research on this topic is still developing, but the future implementation of these diagnostic methods could lead to personalized microbiota-targeted therapies.219 Looking towards the future, further research is required to demonstrate the use of engineered bacteria in the greater context of neurological injury.218 However, we propose that individualized gut microbiota diagnosis and specific genetically engineered bacteria could be an option for personalized microbiota-targeted therapies in the context of neurological illnesses in the future.
7.5 VN Stimulation
There is evidence that gut dysbiosis-induced pro-inflammatory effects are significantly reliant on VN perturbation. In addition, a plethora of recent research has focused on the use of VN stimulation (VNS) in the rehabilitation of stroke and TBI patients.148, 149, 244-250 These studies have shown promising evidence that VNS may rescue decreased gut mucosal integrity by upregulation of enteric glial cells, reduce systemic proinflammatory cytokines and promote recovery.
8 CONCLUSION
The consortium of current scientific literature has demonstrated a strong bidirectional link between gut microbiome dysbiosis and worsening secondary injury post-stroke, SCI and TBI (see summary Figure 6). Furthermore, there is evidence that these deficits may be rescued by timely rejuvenation of the gut microbiome. In addition, the maintenance of a healthy microbiome has been shown to play a protective role in the setting of tumour chemotherapy and immunotherapy. While the future of gut microbiome modulation for neurosurgical therapeutic goals appears increasingly promising, more research is needed to discover the best routes of microbial modification in the setting of specific neurosurgical illnesses.
AUTHOR CONTRIBUTIONS
All authors wrote individual sections. Dr Lucke-Wold organized paper, edited, and provided critical evaluation.
ACKNOWLEDGEMENTS
Not applicable.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The paper was handled by editors and has undergone a rigorous peer-review process. Dr. Brandon Lucke-Wold was not involved in the journal's review of/or decisions related to this manuscript.
ETHICAL APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




