Organization of the antennal lobes in the praying mantis (Tenodera aridifolia)
Funding information: Fyssen Foundation (postdoctoral fellowship to T.C); Canon Foundation (postdoctoral fellowship to T.C.); Japan Society for the Promotion of Science, Grant/Award Number: postdoctoral fellowship PE12038 (to T.C.); Mishima Kaiun Memorial Foundation; Central Research Institute of Fukuoka University, Grant/Award Number 141031.
Abstract
Olfaction in insects plays pivotal roles in searching for food and/or for sexual partners. Although many studies have focused on the olfactory processes of nonpredatory insect species, little is known about those in predatory insects. Here, we investigated the anatomical features of the primary olfactory center (antennal lobes) in an insect predator whose visual system is well developed, the praying mantis Tenodera aridifolia. Both sexes of T. aridifolia were found to possess 54 glomeruli, and each glomerulus was identified based on its location and size. Moreover, we found a sexual dimorphism in three glomeruli (macroglomeruli) located at the entrance of the antennal nerves, which are 15 times bigger in males than their homologs in females. We additionally deduced the target glomeruli of olfactory sensory neurons housed in cognate types of sensilla by degenerating the sensory afferents. The macroglomeruli received sensory inputs from grooved peg sensilla, which are present in a large number at the proximal part of the males' antennae. Furthermore, our findings suggest that glomeruli at the posteriodorsal part of the antennal lobes receive sensory information from putative hygro- and thermosensitive sensilla. The origins of projections connected to the protocerebrum are also discussed. J. Comp. Neurol. 525:1685–1706, 2017. © 2016 Wiley Periodicals, Inc.
1 Introduction
Animals evolved sensory structures adapted to their lifestyles to maximize their interactions with their environment. In insects, olfaction plays pivotal roles in detecting food and seeking for sexual partners (Hansson, 1999). Olfactory information is initially encoded when olfactory molecules bind olfactory receptor neurons (ORNs) that are housed in olfactory sensilla, which are mainly distributed on the antennal surfaces (Hansson, 1999). The encoded information is mediated through ORN axons that run to the proximal part of the antennae (Nishino, Yoritsune, & Mizunami, 2009b; Nishino, Yoritsune, & Mizunami, 2010) and terminate at the primary processing centers in the central nervous system (antennal lobes [ALs]). In the ALs, the axonal terminals are organized in spherical neuropils, called glomeruli (Hansson, 1999). Axons of ORNs expressing the same type of olfactory receptor are believed to converge in the same glomerulus in insects (Vosshall, 2000; Jefferis, Marin, Watts, & Luo, 2002; Couto, Alenius, & Dickson, 2005; Haupt, Sakurai, Namiki, Kazawa, & Kanzaki, 2010). Thus, the number of glomeruli is more and less related to the number of different types of olfactory receptors expressed on ORNs (but see Goldman et al., 2005). Consequently, knowing the glomerular organization of a given species is important for understanding its olfactory capabilities. Furthermore, in insect species that use olfaction to detect sexual partners, males possess a large number of sex pheromone-receptive sensilla on the surface of their antennae (Schafer & Sanchez, 1973, 1976), which are associated with the presence of macroglomeruli (MG), or a macroglomerular complex, that are significantly greater in size than their homologs in females (Rospars, 1983, 1988; Chambille & Rospars, 1985; Strausfeld & Reisenman, 2009; Watanabe, Nishino, Nishikawa, Mizunami, & Yokohari, 2010).
Mantises are insect predators that catch prey based on visual stimuli (Rilling, Mittelstaedt, & Roeder, 1959; Prete, 2004; Yamawaki, 2006), and many studies have focused on their visual systems. However, like other insect species, they also use olfactory cues in their feeding and courtship behaviors. For example, males can determine the mating status of females based on smell (Lelito & Brown, 2008). Furthermore, they can eat pieces of banana and associate this food reward with the odor of banana (Prete, Lum, & Grossman, 1992). Although the morphological features of antennae have been investigated in several species of mantises (Holwell, Barry, & Herberstein, 2007; Allen, Barry, & Holwell, 2012; Carle, Toh, Yamawaki, Watanabe, & Yokohari, 2014a, 2014b), little is known about their olfactory system. In fact, little is known about the olfactory capabilities of insect predators that heavily depend on their visual system to obtain food. Investigating olfaction in predatory insects is crucial to better understand how ecological factors, such as lifestyle (e.g. predation), have influenced the evolution of the olfactory system.
Compared with other insect species, the mantis antennae present a specific sensillar distribution, as observed in T. aridifolia (Carle et al., 2014a) and Sphodromantis lineola (Hurd et al., 2004), which may constitute good model organisms in which to investigate the target glomeruli of olfactory sensory neurons housed in cognate types of sensilla. This atypical sensillar distribution previously led us to divide the flagellum into six different parts (Carle et al., 2014a). Briefly, the proximal parts are equipped with only mechanosensory and hygro-/thermoreceptive sensilla, whereas the distal parts additionally possess olfactory sensilla in females. Moreover, a large number of grooved peg sensilla are present mostly on the proximal parts of male antennae, which are speculated to be their sex-pheromone sensilla (Holwell et al., 2007; Allen et al., 2012; Carle et al., 2014a, 2014b). In the present study, the anatomical organization of ALs in the praying mantis T. aridifolia was investigated to increase our understanding of olfactory processes in predators having a well-developed visual system. Furthermore, the functional organization was investigated using a degeneration method (see Materials and Methods and Vosshall, Wong, & Axel, 2000) to identify the target glomeruli associated with different antennal regions and different types of sensilla.
2 Materials and methods
2.1 Insects and breeding
The experiments were performed using 13 male and 10 female adult praying mantises (T. aridifolia), and 3 male and 5 female 7th instar nymphs. Oothecae were collected in Fukuoka (Japan), and the nymphs obtained were bred to adulthood using previously described methods (Sato & Yamawaki, 2014; Carle, Yamashita, & Yamawaki, 2015). Briefly, the mantises were kept at 25 ± 3 °C with in a 12-hr:12-hr L:D photoperiod (light phase, 9:00–21:00). Until they became 4th instar, nymphs were bred in groups and fed a diet of fruit flies (Drosophila melanogaster). Then they were individually housed and fed crickets (Acheta domesticus; ∼5–20 mm in length) until adulthood.
2.2 Glomerular organization of ALs
2.2.1 Anterograde staining of antennal afferents
The anterograde staining method of antennal afferents used in this study was slightly modified from methods reported previously (Nishino, Nishikawa, Mizunami, Yokohari, 2009a; Nakanishi, Nishino, Watanabe, Yokohari, & Nishikawa, 2010; Watanabe et al., 2010). For this experiment, three male and three female mantises were used. After the animals had been anesthetized by ice, the head, with the antennae, was detached from the body and fixed on a dish in low melting point wax. After fixation, the head was immersed in Ringer's solution (Yager, 1999), and both antennae were cut at the first flagellomere. Then the entire cuticle of the resting parts was removed from the scape, and the antennal nerves were exposed in the Ringer's solution. After removal of the Ringer's solution, the nerves were quickly inserted into a tapered glass electrode containing a 10% aqueous solution of dextran tetramethylrhodamine with biotin (micro-ruby, 3,000 MW, D-7162; Invitrogen, Eugene, OR) and covered with Vaseline (Wako Pure Chemical Industries, Osaka, Japan). All of the prepared samples were then incubated in a humid chamber at a low temperature (4 °C) for 4 days. Then the brain was dissected from the head capsule, fixed with a 4% formaldehyde solution at 4 °C for 3 hr, dehydrated in an ascending ethanol series (from 70% to 100%), and cleared in methyl salicylate.
2.2.2 Confocal laser scanning microscopy
The cleared specimens were observed using a confocal laser scanning microscope (LSM-510; Carl Zeiss, Jena, Germany) with × 10 0.8-NA and ×20 0.8-NA plan apochromatic objectives, and a ×40 1.3-NA plan Neofluar objective. The sensory afferents stained with micro-ruby were visualized using a helium–neon laser with a long-pass emission filter (543 nm), whereas the outline of the brain was visualized by background autofluorescence using an argon laser with a band-pass emission filter (458–514 nm). Optical sections were made at a resolution of 1,024 × 1,024 pixels at 1-μm intervals throughout the entire depth of the ALs following a ventral–dorsal neural axis. Optical image files were converted to TIFF-formatted files with the software LSM Image Browser (RRID: SCR_014344; Carl Zeiss) and ZEN (RRID: SCR_013672; Carl Zeiss).
2.2.3 Image analyses
The contrast and brightness of the figures presented in this study were adjusted using Adobe Photoshop CS6 (RRID: SCR_014199; Adobe Systems, San Jose, CA). The TIFF images were processed using an image processing software (RRID: SCR_014431; Avizo; TGC, Berlin, Germany). Each glomerulus and the brain neuropils were manually outlined in each optical section, which allowed reconstruction of the three-dimensional (3D) aspects of glomeruli and ALs. The volumes of individual glomeruli were measured from reconstructed 3D images using a function (“materialstatistics”) attached to Avizo.
2.2.4 Terminology
We have adopted a body axis for brain representations in all of the figures. The glomeruli were named based on their location, and numbered from the anterior to the posterior side as was previously done in moths, mosquitoes, wasps, and beetles (Sadek, Hansson, Rospars, & Anton, 2002; Smid, Bleeker, van Loon, & Vet, 2003; Greiner, Gadenne, & Anton, 2004; Ignell, Dekker, Ghaninia, & Hansson, 2005; Ghaninia, Hansson, & Ignell, 2007; Kazawa et al., 2009; Hu, Wang, & Sun, 2011). Because the brains of individuals presented slightly different orientations during our observations, we averaged the measurements of loci for every glomerulus based on the position of a landmark glomerulus (Kazawa et al., 2009; see Table 2). Thus the averaged measurements may compensate for the bias in the brains’ orientation and more precisely orient each glomerulus.
2.2.5 Data analyses and statistics
The data were plotted in Excel files (Microsoft), and the statistical analyses were carried out using SPSS version 22 (RRID: SCR_002865; IBM, www.ibm.com/software/analytics/spss). Independent-samples t tests were used to evaluate the volumetric and positional differences of corresponding glomeruli between the sexes.
2.3 Innervation patterns from antennal regions to glomeruli
Because of a specific sensillar distribution on T. aridifolia's antennae (see details in Carle et al., 2014a), the target glomeruli of sensory neurons belonging to a cognate type of sensilla were determined using axonal degeneration. After antennae are cut, injured sensory neurons degenerate within 2 weeks, as seen in fruit flies (Drosophila melanogaster) (Vosshall et al., 2000).
Antennae of adult mantises (10 males and 7 females) were cut and then covered with an ethanol solution (100%) to prevent bacterial infections. Sectioning was done between the different antennal parts. After the antennae were sectioned, the mantises were bred as usual, and the anterograde staining of antennal nerves was performed 2 weeks after cutting of antennae, which consequently revealed the innervation pattern of sensory neurons that were not damaged. Then the stained antennal nerves were observed using LSM.
3 Results
3.1 Glomerular organization of ALs
3.1.1 Projection patterns of antennal sensory afferents
The antennal sensory afferents terminated in four different regions of the mantis' brain: (a) the ALs (Figures 1-7); (b) the protocerebrum (Figure 8): several neurites directly terminated in a region that is located next to and more anteriorly compared with the medial lobe of the mushroom body (MB); (c) the antennal mechanosensory and motor center (AMMC): a bundle of antennal afferents projected and gave off dense terminal arborizations in the neuropil posterior to the AL, the putative AMMC; and (d) the gnathal ganglia (GNG): several branches of the antennal afferents terminated in the anterior region of the GNG.
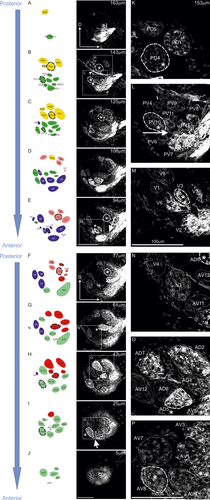
Organization of antennal lobes (ALs) in female mantis (Tenodera aridifolia). (a–j) Serial optical (right) and schematic images (left) of the ALs from posterior (top) to anterior (bottom) side. This figure represents optical sections of confocal images of the ALs after staining the antennal afferents with dextran tetramethylrhodamine plus biotin (micro-ruby), and the schematic representation of glomeruli (left) in female adult mantises from posterior (top) to anterior (bottom) side. In the schematic images, glomeruli presented in different colors indicate different groups. (k–p) Enlargements of images surrounding the square in a–j. For every image, the scale is presented at the bottom of the picture. Each landmark glomerulus is marked with an asterisk and surrounded by a thick white dotted line, whereas the other glomeruli are represented by thin dotted lines. The depth of each optical section from the anterior surface of the ALs is denoted in each picture. PD = posteriodorsal; PV = posterioventral; D = dorsal; V = ventral; AD = anteriodorsal; AV = anterioventral
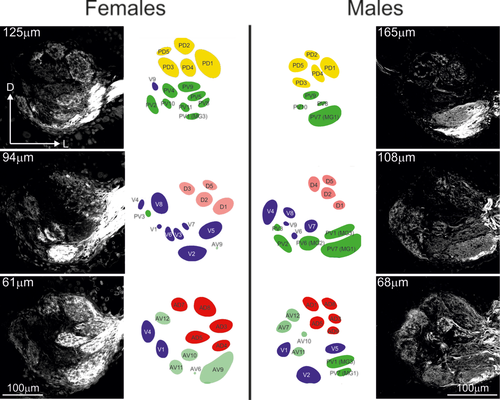
Sexual dimorphism of antennal lobes (ALs). Optical sections of confocal images and a schematic representation of glomeruli from a female (left) and male (right) at three different depths following a posterior (top)/anterior (bottom) axis. PD = posteriodorsal; PV = posterioventral; D = dorsal; V = ventral; AD = anteriodorsal; AV = anterioventral
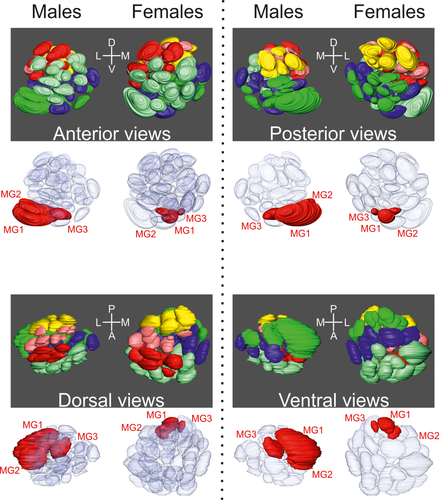
3D representation of the antennal lobes (ALs) from different angles. For each view (anterior, posterior, dorsal, and ventral), we present the 3D reconstruction of ALs from one male and one female. In the top part for each view, each glomerulus is colored depending on its region. In the bottom part, the macroglomeruli in males and their homologs in females are represented in red, whereas other glomeruli are transparent. MG = macroglomeruli
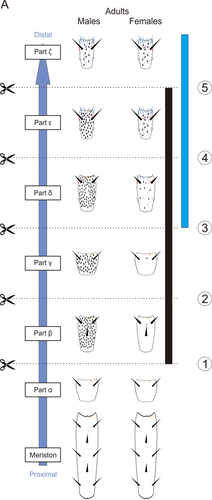
Sensillar distribution in T. aridifolia antennae. In both sexes, each antenna was divided into six different parts based on the sensillar distribution and named from the proximal (bottom: part α) to the distal part (top: part ζ). Males (left) possess a large number of grooved peg sensilla at the parts from β to ε, compared with females (right). Schematic representation of the scissions are represented with numbers. The black bar indicates the parts where grooved-peg sensilla are located in males. The blue bar indicates those where trichoid and basiconic sensilla are located in both sexes
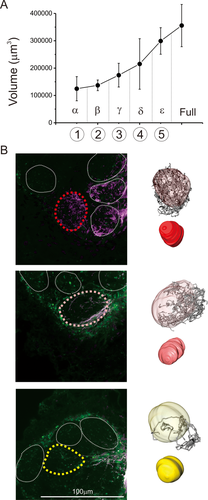
Patterns of innervation after cutting T. aridifolia antennae. (a) Size of macroglomerulus 1 (MG1) in male after the degeneration of antennal nerves resulting from different scissions sites. (b) Optical sections of confocal images of ALs in one female T. aridifolia after having cut the antennae after the part γ. Three different patterns of ORNs innervation were observed, and a 3D representation of the glomeruli is shown on the right. We used an ascending order of colors to represent the level of innervation for each glomerulus: those that did not receive innervations from sensory neurons are represented in white; those surrounded by stained axons are in yellow (bottom); those partially innervated are in pink (middle); and those that were fully innervated are in red (top)
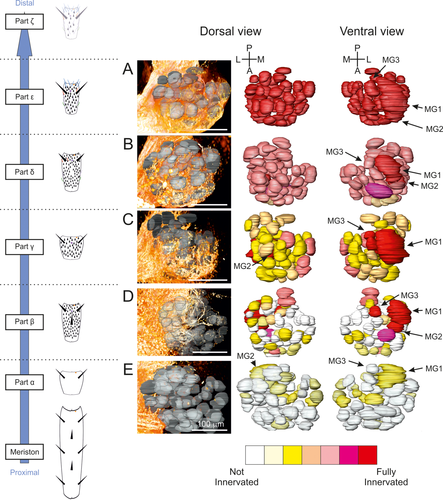
(a–e) Innervation pattern of antennal lobes (ALs) in males T. aridifolia. 3D reconstruction of sensory afferents (orange in the middle panels) in one AL for each scission, after cutting an antenna at the cognate point (dotted line shown in the left schematic drawing). Dorsal and ventral views (right panels) of reconstructed glomeruli labeled with a gradient of color representing an average of the innervation using two specimens at each scission (see Materials and Methods and Table 3). MG = macroglomerulus
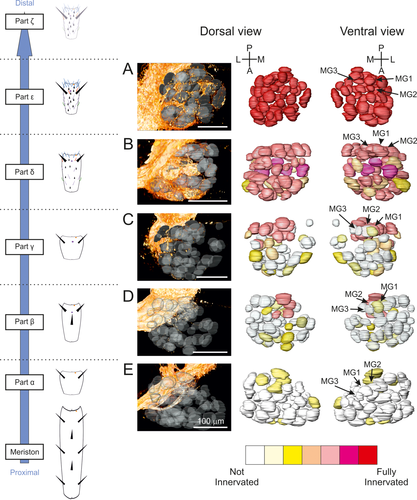
(a–e) Innervation of ALs in female T. aridifolia. See the detailed legend of Figure 6
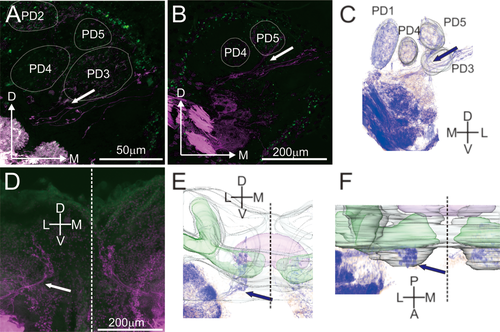
Antennal afferents connecting to the protocerebrum. (a,b) Optical sections of confocal images of antennal afferents connecting to protocerebrum (a, stack: 30 µm; b, stack: 15 µm). Separation point between afferents going to glomeruli and the afferents going to the protocerebrum is indicated by an arrow. Afferents going to the protocerebrum run parallel to the afferents innervating the glomeruli PD3 and PD5. (c) 3D representation of images from b. (d–f) Optical sections (d), from a anterior point of view, of confocal images (stack: 600 µm) and their 3D representations (e and f) of the mantis' brain after staining the antennal nerves with micro-ruby. The afferents going to the protocerebrum are indicated by arrows (d–f). The mushroom bodies and central complex are represented in green and purple, respectively. The dotted lines represent the medial line of the brain
3.1.2 AL organization
In females, anterograde staining of the antennal nerves revealed that antennal sensory afferents bundled into several sensory tracts and terminated in 54 glomeruli (Figure 1). This number was constant among individuals (n = 4 ALs). At the anterior part of ALs, the glomeruli seemed tight, and the axons of sensory neurons formed clustered boutons (Figure 1o,p), whereas the glomeruli were sparser and the axonal terminals were more uniform within each glomerulus in the posterior part (Figure 1k), similar to the anatomical features reported in orthopteran insects (Ignell, Anton, & Hansson, 2001).
Based on landmarks such as the afferents connecting to the protocerebrum, and on the innervation pattern of the sensory tracts, we regionally located the glomeruli and classified them into six different groups in the ALs of the praying mantis T. aridifolia: posteriodorsal (PD), posterioventral (PV), dorsal (D), ventral (V), anteriodorsal (AD), and anterioventral (AV). The groups contained 5, 11, 8, 9, 9, and 12 glomeruli, respectively, and the volume of each glomerulus is listed in Table 1 for adults and 7th instar nymphs. For each group, we have defined landmark glomeruli: PD4, D2, PV10, V3, AD5, and AV8, respectively (see asterisks in Figure 1). Based on their relative positions, in addition to their shapes and sizes, every glomerulus could be unambiguously identified.
| 7th instar | Adults | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||||
| Volume (μm3) | Volume (μm3) | Volume (μm3) | Volume (μm3) | |||||||||||
| n = 3 | n = 5 | n = 3 | n = 4 | Males/ | 7th instar/ | 7th instar/ | ||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | females | p value | adult | p value | adult | p value | |
| PD1 | 38,085.84 | 2,462.56 | 35,511.89 | 5,652.86 | 67,873.54 | 30,760.27 | 46,263.49 | 16,625.06 | 1.47 | 1.78 | 1.30 | |||
| PD2 | 14,289.57 | 3,527.69 | 15,414.23 | 1,434.55 | 24,830.34 | 7,545.43 | 23,238.09 | 6,318.81 | 1.07 | 1.74 | 1.51 | |||
| PD3 | 22,368.98 | 5,023.74 | 24,507.18 | 2,895.34 | 41,454.54 | 24,215.09 | 25,713.30 | 9,467.72 | 1.61 | 1.85 | 1.05 | |||
| PD4 | 23,563.73 | 8,044.26 | 21,220.77 | 2,457.86 | 33,508.44 | 13,384.97 | 34,134.51 | 13,730.12 | 0.98 | 1.42 | 1.61 | |||
| PD5 | 22,600.58 | 5,825.47 | 24,803.16 | 9,644.51 | 56,976.33 | 30,235.08 | 38,640.21 | 21,373.56 | 1.47 | 2.52 | 1.56 | |||
| D1 | 15,930.33 | 2,195.06 | 15,015.42 | 4,861.31 | 31,579.72 | 5,019.71 | 20,447.86 | 7,189.77 | 1.54 | 1.98 | <0.01 | 1.36 | ||
| D2 | 14,767.12 | 719.74 | 17,460.90 | 3,601.76 | 29,854.50 | 11,459.60 | 25,347.40 | 13,562.16 | 1.18 | 2.02 | 1.45 | |||
| D3 | 12,741.84 | 5,585.03 | 11,168.44 | 4,078.37 | 15,141.66 | 8,170.20 | 17,075.55 | 9,646.27 | 0.89 | 1.19 | 1.53 | |||
| D4 | 10,893.20 | 2,482.85 | 14,385.94 | 4,685.74 | 29,475.39 | 3,968.16 | 14,291.04 | 7,082.42 | 2.06 | <0.05 | 2.71 | <0.01 | 0.99 | |
| D5 | 19,205.31 | 3,067.89 | 17,431.01 | 457.81 | 26,800.65 | 19,761.26 | 17,995.76 | 7,026.42 | 1.49 | 1.40 | 1.03 | |||
| D6 | 5,425.75 | 5,621.16 | 6,571.22 | 2,041.15 | 13,443.51 | 9,298.61 | 5,426.49 | 4,657.16 | 2.48 | 2.48 | 0.83 | |||
| D7 | 3,689.12 | 1,111.02 | 7,108.78 | 4,406.15 | 9,583.73 | 2,118.77 | 6,459.95 | 1,978.16 | 1.48 | 2.60 | <0.05 | 0.91 | ||
| D8 | 10,839.76 | 2,609.23 | 8,365.04 | 4,208.98 | 10,807.66 | 6,046.35 | 10,433.84 | 3,161.48 | 1.04 | 1.00 | 1.25 | |||
| PV1 (MG3) | 3,162.08 | 889.56 | 2,758.37 | 1,256.02 | 120,661.05 | 89,545.14 | 6,389.81 | 2,470.84 | 18.88 | <0.05 | 38.16 | 2.32 | <0.05 | |
| PV2 | 14,984.84 | 439.33 | 15,760.76 | 3,875.62 | 61,508.81 | 52,473.51 | 18,385.38 | 2,082.59 | 3.35 | 4.10 | 1.17 | |||
| PV3 | 11,600.39 | 1,913.04 | 16,235.06 | 5,629.44 | 29,957.61 | 25,276.89 | 14,686.56 | 2,802.22 | 2.04 | 2.58 | 0.90 | |||
| PV4 | 12,055.73 | 2,505.43 | 14,880.62 | 3,262.89 | 22,444.75 | 8,359.59 | 21,269.01 | 6,434.00 | 1.06 | 1.86 | 1.43 | |||
| PV5 | 10,955.57 | 2,362.14 | 14,376.79 | 2,450.48 | 22,779.16 | 12,353.89 | 16,710.24 | 8,133.51 | 1.36 | 2.08 | 1.16 | |||
| PV6 (MG2) | 4,149.92 | 346.70 | 2,379.50 | 1,038.99 | 69,918.15 | 32,410.22 | 3,155.85 | 1,548.62 | 22.16 | <0.01 | 16.85 | <0.05 | 1.33 | |
| PV7 (MG1) | 11,228.06 | 866.21 | 11,656.70 | 2,451.81 | 355,683.35 | 134,091.56 | 21,736.95 | 5,837.89 | 16.36 | <0.05 | 31.68 | <0.05 | 1.86 | <0.01 |
| PV8 | 6,088.52 | 1,328.80 | 5,259.68 | 1,454.81 | 13,646.85 | 4,851.79 | 5,661.28 | 3,814.57 | 2.41 | 2.24 | 1.08 | |||
| PV9 | 13,769.16 | 2,720.24 | 19,139.35 | 4,730.15 | 34,463.53 | 18,107.60 | 21,690.90 | 7,012.05 | 1.59 | 2.50 | 1.13 | |||
| PV10 | 9,831.05 | 2,136.91 | 13,070.58 | 4,341.58 | 22,000.94 | 9,855.94 | 17,789.08 | 5,095.72 | 1.24 | 2.24 | 1.36 | |||
| PV11 | 6,744.22 | 3,297.44 | 5,686.87 | 4,094.20 | 11,670.19 | 6,743.15 | 8,127.59 | 4,297.92 | 1.44 | 1.73 | 1.43 | |||
| V1 | 20,696.60 | 2,157.60 | 26,820.33 | 5,483.47 | 32,681.12 | 17,627.76 | 35,538.38 | 9,536.57 | 0.92 | 1.58 | 1.33 | |||
| V2 | 34,440.54 | 7,875.49 | 36,240.89 | 8,206.79 | 46,708.81 | 27,939.59 | 41,881.17 | 7,834.13 | 1.12 | 1.36 | 1.16 | |||
| V3 | 4,638.71 | 886.56 | 5,987.94 | 1,393.23 | 10,293.68 | 4,026.09 | 8,209.08 | 2,663.14 | 1.25 | 2.22 | 1.37 | |||
| V4 | 20,532.97 | 4,491.13 | 26,268.34 | 4,931.21 | 63,706.41 | 31,446.45 | 37,607.05 | 11,967.09 | 1.69 | 3.10 | 1.43 | |||
| V5 | 23,526.99 | 8,142.68 | 30,097.38 | 10,117.31 | 60,255.49 | 33,083.60 | 39,049.27 | 17,494.88 | 1.54 | 2.56 | 1.30 | |||
| V6 | 9,011.64 | 1,203.56 | 10,753.07 | 1,966.38 | 15,892.67 | 9,738.16 | 15,927.97 | 1,598.05 | 1.00 | 1.76 | 1.48 | <0.01 | ||
| V7 | 11,124.12 | 3,192.41 | 11,439.77 | 2,849.52 | 20,323.53 | 7,281.61 | 15,871.64 | 6,694.53 | 1.28 | 1.83 | 1.39 | |||
| V8 | 12,889.93 | 1,362.57 | 13,772.85 | 1,158.26 | 31,029.96 | 19,562.08 | 19,514.59 | 5,313.55 | 1.59 | 2.41 | 1.42 | |||
| V9 | 6,499.08 | 1,919.06 | 10,565.59 | 5,245.31 | 31,386.16 | 33,422.79 | 12,404.85 | 4,901.71 | 2.53 | 4.83 | 1.17 | |||
| AD1 | 15,364.26 | 9,686.49 | 17,498.08 | 10,115.08 | 27,121.74 | 10,954.84 | 24,628.64 | 13,935.42 | 1.10 | 1.77 | 1.41 | |||
| AD2 | 14,007.61 | 4,587.55 | 15,711.77 | 5,614.69 | 28,310.60 | 19,824.73 | 21,410.41 | 8,002.01 | 1.32 | 2.02 | 1.36 | |||
| AD3 | 16,209.75 | 2,320.46 | 10,396.01 | 2,025.28 | 23,452.77 | 11,241.46 | 18,958.99 | 7,259.01 | 1.24 | 1.45 | 1.82 | <0.05 | ||
| AD4 | 10,879.99 | 657.08 | 10,277.12 | 1,949.40 | 16,888.79 | 5,074.25 | 10,864.42 | 3,414.41 | 1.55 | 1.55 | 1.06 | |||
| AD5 | 12,484.48 | 3,040.60 | 11,535.02 | 2,190.84 | 25,776.55 | 8,266.89 | 17,424.30 | 6,948.34 | 1.48 | 2.06 | 1.51 | |||
| AD6 | 10,228.27 | 2,018.79 | 14,378.78 | 1,848.57 | 13,810.77 | 7,062.47 | 11,210.56 | 5,459.46 | 1.23 | 1.35 | 0.78 | |||
| AD7 | 18,438.35 | 3,393.57 | 17,851.12 | 4,854.15 | 33,930.37 | 26,601.94 | 30,590.85 | 7,104.87 | 1.11 | 1.84 | 1.71 | <0.05 | ||
| AD8 | 33,408.34 | 8,043.99 | 26,092.75 | 6,482.56 | 35,874.13 | 11,693.45 | 32,717.84 | 8,722.49 | 1.10 | 1.07 | 1.25 | |||
| AD9 | 4,062.79 | 369.75 | 3,799.37 | 2,748.26 | 11,910.43 | 8,729.11 | 5,755.04 | 1,680.10 | 2.07 | 2.93 | 1.51 | |||
| AV1 | 11,766.38 | 5,366.76 | 11,547.10 | 2,950.54 | 19,592.04 | 7,779.22 | 17,793.53 | 5,530.69 | 1.10 | 1.67 | 1.54 | |||
| AV2 | 10,682.11 | 798.16 | 13,659.16 | 4,780.79 | 31,043.85 | 16,731.37 | 20,581.05 | 5,539.43 | 1.51 | 2.91 | 1.51 | |||
| AV3 | 14,362.16 | 1,028.34 | 13,368.91 | 5,699.33 | 21,651.38 | 10,573.40 | 20,771.80 | 7,115.36 | 1.04 | 1.51 | 1.55 | |||
| AV4 | 14,157.48 | 3,831.04 | 15,739.00 | 2,952.79 | 20,638.22 | 10,026.14 | 21,080.90 | 7,537.17 | 0.98 | 1.46 | 1.34 | |||
| AV5 | 14,237.60 | 1,746.13 | 16,192.02 | 1,798.24 | 31,799.06 | 11,522.14 | 19,566.33 | 8,513.73 | 1.63 | 2.23 | 1.21 | |||
| AV6 | 16,798.18 | 4,964.50 | 17,505.23 | 3,338.94 | 19,720.93 | 11,796.73 | 17,740.86 | 11,118.05 | 1.11 | 1.17 | 1.01 | |||
| AV7 | 14,515.16 | 6,137.81 | 22,299.78 | 5,079.64 | 25,589.34 | 13,316.04 | 27,387.04 | 8,939.07 | 0.93 | 1.76 | 1.23 | |||
| AV8 | 15,460.09 | 5,309.57 | 14,624.91 | 3,016.23 | 31,891.85 | 29,036.58 | 31,028.03 | 13,819.86 | 1.03 | 2.06 | 2.12 | |||
| AV9 | 52,294.91 | 11,049.38 | 55,454.76 | 21,805.73 | 41,338.66 | 41,152.57 | 55,303.50 | 27,155.85 | 0.75 | 0.79 | 1.00 | |||
| AV10 | 15,370.26 | 1,393.71 | 19,078.03 | 5,336.02 | 37,063.08 | 28,847.68 | 34,576.37 | 13,234.50 | 1.07 | 2.41 | 1.81 | |||
| AV11 | 10,876.94 | 2,832.12 | 15,329.44 | 1,817.57 | 22,843.17 | 9,850.71 | 18,980.58 | 3,894.57 | 1.20 | 2.10 | 1.24 | |||
| AV12 | 29,713.52 | 7,603.50 | 31,869.10 | 4,498.28 | 38,319.69 | 14,923.94 | 36,662.60 | 12,496.38 | 1.05 | 1.29 | 1.15 | |||
- Note. Mean size (in µm3) ± SD of every glomerulus in 7th instar nymphs and adults, in males and females. Ratio between males/females at adulthood, and between 7th instar nymphs/adults in males and females are also indicated, with the p values, which were done using independent-samples t tests; significant results are indicated.
3.1.3 Landmark glomeruli
The morphological features of landmark glomeruli were identical among animals and were defined as follows: PD4 was located anteriorly compared with the tract going to the protocerebrum (arrowhead in Figure 1b,k), and surrounded by other glomeruli in the most posterior part of the ALs. PV10 was also located in the posterior part of the ALs and was posterior to the tract going to the protocerebrum (Figure 1b,l). In this glomerular group, PV2 and PV3, which were located more medially than PV10, received sensory inputs from a tract running along the ventral margin of the ALs. Unlike this tract, the tract innervating PV10 ran between other glomeruli located in this region (see arrow in Figure 1b,l). More ventrally, D2 was located in the central point of the dorsal glomerular group (Figure 1e) and was surrounded by other glomeruli. In the ventral side of the ALs (V glomerular group), V3 was located between two well-identified glomeruli, V6 and V7. The tract innervating V3 separated (arrow in Figure 1e,m) and ventrally innervated V2. In the anteriodorsal part of the ALs (AD glomerular group), four glomeruli were aligned and innervated from a common sensory tract, and they were termed AD4, AD5, AD6, and AD7, respectively, from the lateral to the medial side (Figure 1g,h). AD5 was the glomerulus receiving many neurites in the middle part of this alignment (Figure 1g). Finally, AV8 was located in the anterioventralmedial part of the ALs, and its innervating tract ran along the ventral margin of the ALs to reach the medial area (arrow in Figure 1i,p).
3.1.4 Sexual dimorphism
Anterograde staining of the antennal afferents in males (n = 3 ALs) revealed a similar number and organization of glomeruli as found in females. Although differences in the volume of glomeruli did not exceed a ratio of 3.35 between the sexes for the majority of glomeruli, three glomeruli belonging to the PV glomerular group (PV1, PV6, and PV7) were 15 times larger in males than their homologs in females (independent-samples t test, for all values: t > −4.31, p < 0.05; Figures 2 and 3 and Table 1). Therefore, we defined these three glomeruli as MGs and renamed them (PV1 became MG3, PV6 became MG2, and PV7 became MG1) using an ascending terminology based on their size. These glomeruli were all located close to the entrance of the antennal nerves (Figure 3). We also found that the glomerulus D4 was significantly larger (2.06 times) in males (t4.78 = −3,6, p = 0.017). Accompanying differences in the volume of MGs, the relative position of glomeruli, mostly those located around the MG at the entrance of the antennal nerve (AD1, AD8, AV2, AV4, AV5, AV6, AV10, PV3, and PV11), differed between the sexes, even though the glomerular organization was similar (Table 2).
| Adults | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | p value | |||||||||||||
| x-center | y-center | z-center | x-center | y-center | z-center | x-center | y-center | z-center | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| PD1 | 57.20 | 25.86 | −20.75 | 9.88 | 80.18 | 4.64 | 17.01 | 15.00 | −14.12 | 20.15 | 83.98 | 8.08 | – | – | – |
| PD2 | 14.31 | 29.82 | −47.93 | 22.00 | 86.81 | 1.73 | −25.42 | 13.99 | −38.94 | 23.95 | 79.61 | 12.93 | – | – | – |
| PD3 | −28.59 | 7.85 | 10.73 | 23.90 | 92.41 | 15.45 | −64.44 | 20.42 | 7.51 | 29.46 | 70.54 | 15.65 | – | – | – |
| PD4 | 20.24 | 21.28 | −3.12 | 13.27 | 96.36 | 15.68 | −23.15 | 15.03 | 2.56 | 24.55 | 88.79 | 12.91 | – | – | – |
| PD5 | −25.78 | 22.28 | −16.39 | 26.76 | 109.67 | 10.27 | −70.96 | 17.63 | −10.30 | 33.70 | 92.90 | 14.61 | – | – | – |
| D1 | 61.00 | 26.51 | 7.96 | 19.07 | 26.88 | 4.21 | 42.66 | 11.85 | 2.01 | 10.86 | 38.02 | 2.13 | – | – | – |
| D2 | 25.39 | 31.53 | −10.81 | 8.70 | 41.76 | 5.69 | 13.37 | 10.34 | −11.18 | 8.54 | 39.77 | 6.29 | – | – | – |
| D3 | −20.82 | 8.95 | −0.34 | 16.89 | 39.08 | 7.75 | −30.28 | 14.81 | −4.14 | 27.11 | 32.97 | 8.11 | – | – | – |
| D4 | −5.96 | 31.11 | −44.11 | 13.52 | 42.75 | 2.22 | −24.78 | 10.72 | −35.02 | 21.64 | 37.05 | 9.12 | – | – | – |
| D5 | 47.47 | 30.08 | −41.28 | 3.44 | 42.37 | 11.00 | 16.53 | 16.56 | −34.83 | 13.17 | 53.83 | 4.30 | – | – | – |
| D6 | −17.82 | 20.35 | −47.87 | 33.10 | 70.77 | 3.67 | −32.34 | 15.51 | −46.58 | 18.26 | 55.75 | 8.54 | – | – | – |
| D7 | −44.11 | 13.56 | −27.34 | 29.08 | 68.70 | 5.07 | −61.67 | 6.20 | −30.55 | 24.56 | 55.62 | 8.92 | – | – | – |
| D8 | 64.07 | 37.60 | −46.14 | 3.13 | 62.17 | 11.35 | 29.52 | 12.22 | −39.22 | 23.09 | 70.63 | 7.15 | – | – | – |
| PV1 | 57.39 | 7.66 | 79.49 | 31.93 | 29.06 | 30.95 | −7.99 | 4.37 | 77.35 | 20.14 | 55.47 | 22.30 | – | – | – |
| PV2 | −61.13 | 25.70 | 125.04 | 21.92 | 53.83 | 17.30 | −93.31 | 8.16 | 78.51 | 31.35 | 34.03 | 27.11 | – | – | – |
| PV3 | −88.74 | 26.86 | 87.44 | 11.37 | 75.54 | 26.59 | −110.19 | 12.40 | 48.62 | 33.33 | 31.40 | 23.54 | – | <0.05 | – |
| PV4 | −18.15 | 4.55 | 49.39 | 3.09 | 71.44 | 31.07 | −48.15 | 9.78 | 41.17 | 25.48 | 54.78 | 19.63 | – | – | – |
| PV5 | 26.58 | 10.68 | 51.59 | 12.62 | 66.93 | 28.82 | −1.34 | 9.88 | 43.53 | 18.98 | 62.99 | 11.76 | – | – | – |
| PV6 | −21.20 | 17.51 | 111.16 | 16.14 | 66.56 | 42.52 | −43.30 | 6.27 | 87.75 | 26.89 | 57.03 | 30.45 | – | – | – |
| PV7 | 45.71 | 12.88 | 110.59 | 38.47 | 68.76 | 42.78 | −21.34 | 5.57 | 98.05 | 26.48 | 67.74 | 23.93 | – | – | – |
| PV8 | 45.66 | 23.64 | 56.39 | 31.62 | 74.83 | 35.00 | 3.10 | 14.22 | 61.09 | 24.96 | 70.15 | 17.75 | – | – | – |
| PV9 | 11.19 | 17.64 | 39.82 | 6.76 | 91.99 | 28.71 | −25.37 | 12.48 | 32.59 | 23.33 | 69.49 | 15.39 | – | – | – |
| PV10 | −28.15 | 14.93 | 82.59 | 4.12 | 90.38 | 40.17 | −59.74 | 6.92 | 71.16 | 31.30 | 65.46 | 22.30 | – | – | – |
| PV11 | 3.02 | 9.17 | 77.63 | 16.22 | 88.06 | 28.27 | −25.81 | 6.84 | 68.39 | 25.89 | 72.11 | 13.21 | <0.05 | – | – |
| V1 | −77.73 | 22.31 | 67.44 | 8.45 | 18.85 | 21.29 | −75.54 | 7.94 | 55.64 | 20.46 | −5.63 | 18.77 | – | – | – |
| V2 | −9.45 | 20.46 | 113.20 | 25.97 | 1.23 | 14.00 | −6.00 | 10.20 | 88.21 | 11.59 | 18.59 | 19.80 | – | – | – |
| V3 | −39.05 | 12.67 | 57.40 | 0.90 | 27.50 | 25.12 | −43.20 | 17.22 | 56.15 | 12.49 | 14.60 | 15.58 | – | – | – |
| V4 | −109.89 | 29.78 | 46.93 | 23.49 | 48.74 | 18.10 | −111.10 | 13.33 | 27.07 | 26.17 | −0.50 | 16.25 | – | – | – |
| V5 | 45.09 | 10.65 | 50.50 | 22.13 | 20.57 | 18.60 | 23.68 | 5.21 | 44.94 | 10.62 | 37.50 | 10.47 | – | – | – |
| V6 | −48.47 | 16.73 | 97.95 | 4.12 | 36.67 | 26.57 | −60.89 | 4.91 | 74.87 | 25.27 | 26.96 | 21.14 | – | – | – |
| V7 | −6.81 | 7.93 | 64.94 | 12.09 | 42.67 | 24.06 | −24.06 | 4.10 | 55.85 | 18.48 | 36.82 | 18.35 | – | – | – |
| V8 | −72.27 | 16.38 | 30.93 | 28.01 | 57.59 | 13.20 | −86.16 | 11.42 | 16.70 | 22.17 | 24.70 | 16.68 | – | – | – |
| V9 | −74.25 | 27.43 | 63.08 | 19.86 | 67.33 | 30.74 | −87.09 | 9.43 | 41.73 | 29.34 | 29.81 | 26.72 | – | – | – |
| AD1 | −12.28 | 9.16 | −41.95 | 20.58 | −17.16 | 12.95 | −16.47 | 7.20 | −40.03 | 8.13 | −26.52 | 7.65 | – | – | <0.05 |
| AD2 | 36.26 | 18.53 | −57.75 | 10.03 | −12.28 | 14.39 | 25.05 | 17.09 | −53.71 | 15.81 | −4.37 | 10.74 | – | – | – |
| AD3 | 48.49 | 12.01 | −16.56 | 8.79 | −6.34 | 7.13 | 32.83 | 14.94 | −21.05 | 7.05 | −3.48 | 9.13 | – | – | – |
| AD4 | 45.64 | 9.74 | 28.90 | 16.63 | −16.60 | 4.82 | 38.55 | 8.84 | 15.22 | 6.75 | 1.20 | 6.82 | – | – | – |
| AD5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – | – | – |
| AD6 | −30.14 | 1.72 | −9.71 | 12.39 | 14.37 | 8.26 | −24.36 | 2.59 | −15.08 | 2.99 | 0.84 | 4.95 | – | – | – |
| AD7 | −34.34 | 10.64 | −48.43 | 21.25 | 9.22 | 8.59 | −45.32 | 13.70 | −40.59 | 9.23 | −0.23 | 7.43 | – | – | – |
| AD8 | 31.74 | 21.84 | −54.62 | 4.84 | 12.41 | 13.69 | 14.40 | 11.53 | −49.68 | 4.83 | 17.27 | 7.26 | – | – | <0.05 |
| AD9 | 46.29 | 8.91 | 11.64 | 17.78 | 8.40 | 6.04 | 28.01 | 6.92 | 9.15 | 6.20 | 18.10 | 5.65 | – | – | – |
| AV1 | −6.66 | 4.48 | 19.98 | 15.78 | −42.12 | 6.79 | 4.33 | 12.53 | 11.54 | 12.61 | −48.31 | 12.13 | – | – | – |
| AV2 | 2.19 | 20.84 | 42.64 | 7.42 | −45.98 | 4.71 | 10.95 | 22.35 | 42.71 | 17.15 | −43.37 | 11.09 | – | – | <0.05 |
| AV3 | −11.84 | 11.84 | −7.70 | 18.63 | −31.39 | 9.29 | −2.30 | 10.47 | −10.47 | 14.29 | −39.75 | 8.73 | – | – | – |
| AV4 | −48.05 | 10.71 | 37.89 | 7.92 | −33.10 | 8.20 | −24.90 | 18.62 | 36.51 | 11.12 | −47.78 | 14.98 | – | – | <0.05 |
| AV5 | 34.70 | 13.28 | −2.42 | 12.21 | −33.72 | 8.43 | 36.79 | 6.85 | −13.09 | 17.51 | −27.93 | 9.00 | – | – | <0.05 |
| AV6 | 16.43 | 33.05 | 66.19 | 16.97 | −43.34 | 10.22 | 21.03 | 16.26 | 50.91 | 10.93 | −25.71 | 12.22 | – | – | <0.05 |
| AV7 | −81.56 | 15.82 | 14.02 | 18.10 | −9.06 | 11.09 | −63.86 | 10.55 | −0.89 | 10.85 | −43.62 | 5.00 | – | – | – |
| AV8 | −83.14 | 25.12 | 51.29 | 7.65 | −12.00 | 12.09 | −65.64 | 19.53 | 41.10 | 11.42 | −40.00 | 12.87 | – | – | – |
| AV9 | 0.60 | 12.75 | 96.07 | 10.71 | −26.23 | 9.21 | 41.36 | 9.47 | 58.67 | 11.70 | −6.97 | 16.03 | – | – | – |
| AV10 | −23.52 | 8.64 | 36.25 | 6.43 | −9.20 | 10.51 | −18.21 | 10.89 | 27.79 | 1.71 | −17.64 | 6.95 | – | – | <0.05 |
| AV11 | −41.61 | 17.35 | 74.98 | 5.03 | −3.00 | 19.68 | −35.78 | 13.98 | 62.88 | 6.66 | −10.30 | 19.06 | – | – | – |
| AV12 | −71.85 | 15.41 | −7.15 | 21.81 | 13.94 | 4.36 | −62.95 | 13.05 | −6.42 | 13.58 | −14.39 | 5.82 | – | – | – |
- Note. The location for each glomerulus is expressed as mean ± SD and was based on the location of the glomerulus AD5. The p values were determined using independent-samples t tests and significant results are indicated. A dash indicates that significant results were not found.
To confirm the presence of three MGs, we investigated the ALs in 7th instar nymphs. We found that 7th instar nymphs possess a similar glomerular organization as female adults, with 54 glomeruli in both sexes. During the glomerular development from the 7th instar to adulthood (n = 3 in males and n = 4 in females), the three MGs (PV7 [MG1], PV6 [MG2], and PV1 [MG3]) increased in volume more than 15 times in males (Table 1). PV7 (MG1) and PV1 (MG3) also significantly increased in females, by 1.86 and 2.32 times, respectively (for both values: t < −2.89, p < 0.05). The volumes of several other glomeruli also significantly increased from the last instar to adulthood in both sexes, but the increases were small ( < five times) in comparison with those of the three MGs (Table 1). Among these increases, we noticed that D1, D4, and D7 in males (for all values, t < −4.27, p < 0.05) and V6, AD3, and AD7 in females (for all values, t < −2.39, p < 0.05) slightly but significantly increased in volume.
3.2 Pattern of innervation from antennal regions to glomeruli
3.2.1 Degeneration of sensory afferents
T. aridifolia possess a specific sensillar distribution on their antennae (Carle et al., 2014a). Because of this specific sensillar distribution, we had previously divided the antennae into six different parts from α (proximal) to ζ (distal) (Figure 4). The part α possesses chaetic, campaniform, and coelocapitular sensilla, and is deprived of olfactory sensilla in both sexes; males present a large number of grooved peg sensilla from the part β to ɛ; basiconic and trichoid sensilla are present on the parts δ, ɛ, and ζ in both sexes. Because the parts α, β, and γ do not possess any olfactory sensilla in females, anterograde staining performed after cutting the females' antennae proximal to part δ (cuts 1, 2, and 3; Figure 4) revealed the innervation pattern of sensory neurons only housed in chaetic, campaniform, and coelocapitular sensilla. Moreover, when the males' antennae are cut proximal to part δ, the sensory neurons housed in grooved peg sensilla are additionally stained because of their abundance on parts β and γ. By subtracting the results of one sex from the other, we can discern the glomeruli receiving sensory inputs from grooved peg sensilla located on the proximal parts of males' antennae.
After the neural degeneration caused by cutting the antennae at different positions, the size of MG1 (because of its large size in males) was measured to evaluate the degeneration processes of sensory afferents. A correlation between the size of MG1 and the size of antennae that remained was found (Figure 5a), indicating that a period of 2 weeks is sufficient for antennal afferents to degenerate.
We found different levels of glomerular innervation from antennal sensory neurons that had not degenerated. Therefore, we defined the degree of innervation by assigning each glomerulus a color (Figure 5b and Table 3). In Figure 4c, the glomeruli that did not receive innervations from sensory neurons are represented in white; those surrounded by stained axons are in yellow; those partially innervated are in pink; and those fully innervated are in red (Figure 5b). Using two specimens per sex and per part cut, the average innervations are shown using an increasing color gradient (Table 3) in the 3D-reconstructed images for both sexes (Figures 6 and 7).
| Males | Females | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha | Beta | Gamma | Delta | Epsilon | Alpha | Beta | Gamma | Delta | Epsilon | |||||||||||||||||||||
| #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | #1 | #2 | Avg | |
| PD1 | ||||||||||||||||||||||||||||||
| PD2 | ||||||||||||||||||||||||||||||
| PD3 | ||||||||||||||||||||||||||||||
| PD4 | ||||||||||||||||||||||||||||||
| PD5 | ||||||||||||||||||||||||||||||
| D1 | ||||||||||||||||||||||||||||||
| D2 | ||||||||||||||||||||||||||||||
| D3 | ||||||||||||||||||||||||||||||
| D4 | ||||||||||||||||||||||||||||||
| D5 | ||||||||||||||||||||||||||||||
| D6 | ||||||||||||||||||||||||||||||
| D7 | ||||||||||||||||||||||||||||||
| D8 | ||||||||||||||||||||||||||||||
| PV1 | ||||||||||||||||||||||||||||||
| PV2 | ||||||||||||||||||||||||||||||
| PV3 | ||||||||||||||||||||||||||||||
| PV4 | ||||||||||||||||||||||||||||||
| PV5 | ||||||||||||||||||||||||||||||
| PV6 | ||||||||||||||||||||||||||||||
| PV7 | ||||||||||||||||||||||||||||||
| PV8 | ||||||||||||||||||||||||||||||
| PV9 | ||||||||||||||||||||||||||||||
| PV10 | ||||||||||||||||||||||||||||||
| PV11 | ||||||||||||||||||||||||||||||
| V1 | ||||||||||||||||||||||||||||||
| V2 | ||||||||||||||||||||||||||||||
| V3 | ||||||||||||||||||||||||||||||
| V4 | ||||||||||||||||||||||||||||||
| V5 | ||||||||||||||||||||||||||||||
| V6 | ||||||||||||||||||||||||||||||
| V7 | ||||||||||||||||||||||||||||||
| V8 | ||||||||||||||||||||||||||||||
| V9 | ||||||||||||||||||||||||||||||
| AD1 | ||||||||||||||||||||||||||||||
| AD2 | ||||||||||||||||||||||||||||||
| AD3 | ||||||||||||||||||||||||||||||
| AD4 | ||||||||||||||||||||||||||||||
| AD5 | ||||||||||||||||||||||||||||||
| AD6 | ||||||||||||||||||||||||||||||
| AD7 | ||||||||||||||||||||||||||||||
| AD8 | ||||||||||||||||||||||||||||||
| AD9 | ||||||||||||||||||||||||||||||
| AV1 | ||||||||||||||||||||||||||||||
| AV2 | ||||||||||||||||||||||||||||||
| AV3 | ||||||||||||||||||||||||||||||
| AV4 | ||||||||||||||||||||||||||||||
| AV5 | ||||||||||||||||||||||||||||||
| AV6 | ||||||||||||||||||||||||||||||
| AV7 | ||||||||||||||||||||||||||||||
| AV8 | ||||||||||||||||||||||||||||||
| AV9 | ||||||||||||||||||||||||||||||
| AV10 | ||||||||||||||||||||||||||||||
| AV11 | ||||||||||||||||||||||||||||||
| AV12 | ||||||||||||||||||||||||||||||
- Note. This table represents the innervation patterns observed for each glomerulus following our gradient of color when antennae were cut distal from part α (cut 1), β (cut 2), γ (cut 3), δ (cut 4), and ε (cut 5), The average (Avg) is also represented for each scission in males (left) and females (right).
3.2.2 Sensillar type-dependent glomerular organization
After the neural degeneration caused by cutting the antennae between the parts α and β in both sexes, a few sensory neurons still projected to the ALs (Figures 6e and 7e), although the remaining antennae of both sexes are not equipped with any types of olfactory sensilla (Figure 4). However, the resting axons surrounded, but did not enter, the glomeruli, and, consequently, the receiving glomeruli were assigned the color yellow (bottom panels in Figures 6e and 7e). Because isolated axons might have survived the degeneration process, the glomeruli represented as yellow have been excluded from the following data analysis.
In both males and females in which the antennae had been cut distal to the part δ (Figures 6b and 7b), every glomerulus received sensory innervations, and a reduction of innervation was found at most glomeruli in both males and females. This suggested that the ORNs present from the part δ to the tip end of antennae innervate every glomerulus. This perfectly correlates with the pattern of sensillar distribution along the antennae, showing that every type of sensilla is present on the parts δ, ε, and ζ (Figures 6b and 7b).
Cutting the antennae between the parts γ and δ (cut 3; Figure 4) caused a reduction in the innervation of most glomeruli in both sexes. In particular, the glomeruli in the anterior region did not receive any sensory innervations in either sex (yellow and white glomeruli in Figures 6c and 7c). The trichoid and basiconic sensilla being present at the part δ and at the more distal parts of antennae in both sexes may indicate that the glomeruli located in the anterior region mainly receive sensory inputs from these types of sensilla. However, the three MGs (MG1/PV7, MG2/PV6, and MG3/PV1) received many sensory innervations when the antennae were cut after the part β (Figure 6D) or after the part γ in males (Figure 6c), but not their homologs in females (Figure 7d). Since grooved peg sensilla are abundantly distributed on the part β in males but not in females (Carle et al., 2014a), these results indicated that the ORNs housed in grooved peg sensilla and located on the parts β and γ directly innervate the MGs in males. Finally, although female mantises do not possess olfactory sensilla on the most proximal parts of antennae (Carle et al., 2014a), cutting the antennae between the parts β and γ (cut 2; Figure 4) or between the parts γ and δ (cut 3; Figure 4) revealed neurites innervating glomeruli in the dorsal parts of the ALs, in the PD glomerular group, especially in PD3 and PD5. Because the females possess only coelocapitular sensilla located on the most proximal parts (α to γ) of their antennae, it is likely that these glomeruli are innervated by neurons located at the base of coelocapitular sensilla.
3.2.3 Sensory afferents innervating the protocerebrum
Although the proximal parts of antennae are totally free of olfactory sensilla in females (Carle et al., 2014a), neurites reaching the protocerebrum, running parallel to neurites innervating glomeruli PD3 and PD5, were observed (Figs. 8a–c). Passing through the ALs, they terminate in a region that is more anterior and dorsal than the mushroom bodies (Figure 8d–f). However, cutting the antennae between the parts α and β (cut 1; Figure 4) and staining the antennal nerves in both sexes did not reveal this tract (Figures 6e and 7e).
4 Discussion
Our study constitutes the first investigation of the anatomical organization of primary olfactory centers in mantis. Although these insect predators are famous for their visual system, they possess 54 glomeruli, similar to crickets and fruit flies (Stocker, Lienhard, Borst, & Fischbach, 1990; Laissue et al., 1999; Ignell et al., 2001; Yoritsune & Aonuma, 2012), and many more than another insect predator, the dragonfly (Rebora et al., 2013). Moreover, our anatomical findings on the projection patterns from antennal regions resulted in a new understanding of the functional aspects of mantis ALs. Specifically, we identified target glomeruli from ORNs located in cognate types of antennal sensilla. Additionally, we highlighted the fact that three MGs selectively receive afferents from ORNs housed in grooved peg sensilla, likely constituting the sex-pheromone processing pathway in T. aridifolia.
4.1 AL organization
In the present study, 54 glomeruli were identified in praying mantis (T. aridifolia). This number is lower compared with that in bees, wasps, cockroaches, and ants, which possess more than 140 glomeruli (Arnold, Masson, & Budharugsa, 1985; Smid et al., 2003; Kleineidam, Obermayer, Halbich, & Rössler, 2005; Zube, Kleineidam, Kirschner, Neef, & Rössler, 2008; Nakanishi et al., 2010; Watanabe et al., 2010; Galizia, Eisenhardt, & Giurfa, 2011; Roselino, Hrncir, da Cruz Landim, Giurfa, & Sandoz, 2015), or the thousands of microglomeruli found in locusts (Anton & Hansson, 1996; Ignell et al., 2001). However, the number of glomeruli in T. aridifolia is greater than the number in another insect species having predatory habits, the dragonfly (Libellula depressa), which possesses only two glomeruli, an anterioventral lobe and a larger posteriodorsal lobe (Rebora et al., 2013). Although mantises and dragonflies have well-developed visual systems (Rossel, 1996; from Rebora, Piersanti, & Gaino, 2008), the different numbers of glomeruli in these species suggest that olfaction plays a more important role in T. aridifolia.
The number of glomeruli in T. aridifolia is similar to that in fruit flies, moths, beetles, mosquitoes, and crickets (Rospars, 1983; Stocker et al., 1990; Rospars & Hildebrand, 1992; Laissue et al., 1999; Ignell et al., 2001; Berg, Galizia, & Brandt, 2002; Greiner et al., 2004; Skiri, Rø, Berg, & Mustaparta, 2005; Ghaninia et al., 2007; Dreyer et al., 2010; Lefaldli, Kvello, & Mustaparta, 2010; Hu et al., 2011; Yoritsune and Aonuma, 2012). Assuming a 1:1 relationship between glomeruli and olfactory receptors, T. aridifolia may possess a number of olfactory receptors similar to D. melanogaster, and consequently might have similar discriminatory capabilities. In such a case, it would not be surprising that praying mantises are able to detect and rely on odors, such as banana, to find rewards (Prete et al., 1992), even though they are insect predators. What role(s) olfaction plays (except in the search for sexual partners) in an insect predator that catches its prey primarily using its visual system (Roeder, 1960) needs to be elucidated in future.
One explanation might lie in the fact that the mantises use olfactory cues to find prolific hunting sites or prey. The praying mantis is an ambush predator mostly waiting for prey (Roeder, 1960). In contrast to dragonflies that search for and hunt prey, the rate of capture in mantises partially depends on the number of prey that surrounds them. Therefore, they have to find prolific sites where prey are in abundance, to satisfy their feeding needs, a strategy used in spiders (Morse, 1986). To this purpose, they might rely on odor of prey, on odors that attract prey (e.g., fruits as odor of banana), or on a combination of both. Although this question has to be investigated in future, it might explain the difference in the olfactory system between the dragonfly and the praying mantis.
4.2 Macroglomeruli
Male T. aridifolia possess three MGs. These MGs are located at the entrance of the antennal nerve and are 15 times larger than their homologs in females, and their size increases more than 15 times during the last molting. These morphological features correspond to MGs that mediate sex-pheromone information in other insect species (Christensen, Harrow, Cuzzocrea, Randolph, & Hildebrand, 1995; Christensen & Hildebrand, 2002; Nishino, Nishikawa, Yokohari, & Mizunami, 2005; Nishino et al., 2009b). Furthermore, the ORNs housed in grooved peg sensilla, which are present in large numbers in adult males, directly innervate these MGs. Thus, with the awareness that electrophysiological studies may confirm our assumption, our study strongly suggests that sex-pheromone information is received by the ORNs in grooved peg sensilla and mediated through these three MGs.
The presence of three MGs in T. aridifolia suggests that the sex pheromones emitted from females possess three different components. Indeed, the number of MGs present in males directly reflects the number of different components of the sex pheromones in moths (Hansson, Ljungberg, Hallberg, & Lofstedt, 1992; Ochieng, Anderson, & Hansson, 1995; Berg et al., 2002; Greiner et al., 2004) and in cockroaches (Burrows, Boeckh, & Esslen, 1982; Boeckh & Selsam, 1984; Nishino et al., 2009b). More specifically, the major sex-pheromone component is related to the largest glomeruli, whereas subcomponents are mediated through the surrounding glomeruli (Christensen et al., 1995; Hansson, Almaas, & Anton, 1995; Berg, Mustaparta, Almaas, Bjaalie, & Mustaparta, 1998; Vickers, Christensen, & Hildebrand, 1998; Vickers & Christensen, 2003; Skiri et al., 2005). It is interesting to note that two main components (and “also may include a trace of” a third component) have been identified as sex pheromones in a different species of praying mantis, S. lineola (Hurd et al., 2004). However, the relationship between sex pheromones and the physiological processes in MGs needs to be clarified in future in T. aridifolia and in mantises in general.
4.3 Sensillar type–dependent glomerular organization
In insects, ORNs housed in different types of sensilla express different types of olfactory receptors and exhibit different olfactory response spectra (Fujimura, Yokohari, Tateda, 1991; de Bruyne, Foster, & Carlson, 2001; Couto et al., 2005). Elucidation of the sensillar type–dependent glomerular organization is important to understand the functional organization of ALs (Anton & Rospars, 2004; Couto et al., 2005; Watanabe, Haupt, Nishino, Nishikawa, & Yokohari,, 2012; Kropf, Kelber, Bieringer, & Rössler, 2014). Based on the non-uniform distribution of sensilla along the antenna of T. aridifolia, the relationship between sensillar types and glomerular groups was revealed using degenerations. This method may become a tool to study the functional organization of the ALs in many insect species since non-uniform distribution of olfactory sensilla is often observed across insect species (fruit fly: de Bruyne et al., 2001; Couto et al., 2005; ant: Nakanishi, Nishino, Watanabe, Yokohari, & Nishikawa, 2009; honey bee: Nishino et al., 2009a; Kropf et al., 2014).
The degeneration experiments showed that glomeruli are organized into groups depending on the sensillar type. As in the cockroach Periplaneta americana (Watanabe et al., 2012), ORNs and basiconic sensilla innervated the dorsal and anterior parts of ALs, whereas ORNs in trichoid and grooved peg sensilla projected to the ventral and posterior parts of ALs. In other insect species, ORNs belonging to the same type of sensilla project to the same region in the ALs (mosquitoes: Anton & Rospars, 2004; fruit flies: Couto et al., 2005). Therefore, the sensillar type–dependent organization of glomeruli is a common architecture in the primary olfactory centers of insects. Because cockroaches and mantises are phylogenetically close (Misof et al., 2014), we might expect that the innervation patterns of ORNs in ALs have a common origin. Moreover, ORNs in different types of sensilla differ in their response spectra in cockroach (Fujimura et al., 1991), indicating that each glomerular group is functionally segregated. Further physiological studies are required in mantis for a better comprehension of the functional aspects of each glomerular group.
We also identified glomeruli that may process hygro- and thermosensory information in T. aridifolia. The degeneration experiments revealed that several glomeruli located at the posterior regions of ALs selectively receive sensory inputs from the proximal part of antennae, where coelocapitular sensilla are located. Based on the anatomical features of this type of sensilla in mantis, we putatively defined this type as hygro- and thermosensory sensilla (Carle et al., 2014a). As for the sex-pheromone pathway, the function of these glomeruli has to be confirmed with electrophysiology in T. aridifolia. Nevertheless, in other insect species also, such as cockroaches (Nishikawa, Yokohari, & Ishibashi, 1995), ants (Nakanishi et al., 2010), and fruit flies (Hamada et al., 2008; Enjin et al., 2016), glomeruli located on the posterior part of the ALs also process hygro- and thermosensory modalities. Therefore, the location and physiological characteristics of these glomeruli might be evolutionarily conserved among insect species.
4.4 Sensory afferents innervating the protocerebrum
The presence of a single tract crossing the ALs and connecting directly to the protocerebrum was observed in T. aridifolia, as in other insect species, Palaeoptera, and Neoptera species (moth: Kent & Hildebrand, 1987; cockroach: Watanabe et al., 2010; cricket: Yoritsune & Aonuma, 2012; locust: Bräunig & Krumpholz, 2013; dragonfly: Rebora et al., 2013; homopteran species: Stacconi, Hansson, Rybak, & Romani, 2014). Specifically, they appear to terminate in a region defined as the crepine and/or the superior medial protocerebrum in D. melanogaster (Ito et al., 2014). Although this tract is present in many insect species, its function and origin is still subject to debate. It has been supposed to mediate mechanosensory information and, in particular, proprioceptive information from the proximal part of the antennae (scapus) (Rospars, 1988; Yoritsune & Aonuma, 2012). However, this tract was absent when the antennae were cut distal to the part α and did not originate from the scapus and/or pedicel (Figs. 6E and 7E), which invalidates such a hypothesis. In the present study, this tract is visible when scissions were made distally from the part β of females, which corresponds to the pattern of distribution of coelocapitular sensilla (cf. Carle et al., 2014a). However, we cannot exclude that nervous degeneration was not complete because of a diffuse innervation pattern after antennal degeneration. Moreover, the tract seems to be associated with and run in parallel with axons that terminate in glomeruli located at the posteriodorsal part of the ALs, a region known to be related to hygrosensitive, thermosensitive, or CO2-associated information (Nishikawa et al., 1995; Stange & Stowe, 1999; Nishino et al., 2009a; Nakanishi et al., 2010). Although the origin of this tract was not precisely defined, our observations strongly suggested that it might be involved in hygro- and thermosensory modalities in concordance with the findings of Bräuning & Krumpholz (2013) and Nishikawa et al. (1995). Indeed, Nishikawa et al. (1995) showed in the cockroach that the sensory neurons housed in capitular sensilla, which are homologs of the coelocapitular sensilla in the mantis, terminate at the ALs only. In addition, Bräuning and Krumpholz (2013) suggested that the tract going to the protocerebrum originated from internal receptors that process thermosensory modalities. Consequently, although without electrophysiological evidence, we assume that this tract might correspond to axons of neurons encoding thermosensitive information located inside the antennal cuticle.
Acknowledgments
This work was supported by postdoctoral fellowships to TC from the Fyssen Foundation, the Canon Foundation, and the Japan Society for the Promotion of Science (PE12038), as well as a grant from the Mishima Kaiun Memorial Foundation and the Central Research Institute of Fukuoka University (141031). We especially thank Sayaka Umiguchi for her invaluable help and Dr. Hiroshi Nishino for his invaluable advice.
Conflict of interest
There are no conflicts of interest.
Author contributions
All authors had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TC, YY, and FM. Acquisition of data: TC. Analysis and interpretation of data: TC, YY, HW, and FY. Drafting of the article: TC, YY, HW, and FY.