Antibacterial, Anti-Biofouling, and Antioxidant Prospects of Metal-Based Nanomaterials
Abstract
Three metallic nanomaterials, i.e., iron, copper, and bimetallic iron–copper were easily synthesized using a wet-chemical method and were properly characterized. The materials were tested against two bacterial strains, Staphylococcus aureus and Escherichia coli in order to evaluate their antibacterial activity, as well as their biofilm formation inhibition properties. The total inhibition of bacterial growth, at low µg/mL concentrations, implies the satisfactory antibacterial activity of the nanomaterials against bacteria. Between the two bacterial strains used, E. coli is more susceptible to the effect of the nanomaterials than S. aureus. However, the results for the respective biofilms are significantly different since S. aureus biofilm formation is more easily inhibited. There are differences in the way of action against the two different bacteria and diverse mechanisms of action are proposed to justify them. Furthermore, positive 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical tests demonstrated that the nanomaterials are free-radical scavengers while TBARS (thiobarbituric acid reactive substances) tests evidenced their lipid oxidation inhibition properties, although at high concentration levels. Copper nanoparticles proved to be the most effective of the three materials tested, against DPPH radicals and lipid peroxidation followed by the Fe–Cu and lastly by the Fe nanoparticles. The intrinsic magnetic properties of iron-based materials can provide long-term benefits for antibacterial or anti-biofouling treatment in water purification systems as well as for antioxidant activity purposes.
Abbreviations
-
- AAS
-
- atomic absorption spectrometry
-
- AE
-
- antioxidant efficiency
-
- BET
-
- Brunauer–Emmett–Teller
-
- BHT
-
- 3,5-di-tert-4-butylhydroxytoluene
-
- DDW
-
- double-distilled water
-
- DPPH
-
- 1,1-diphenyl-2-picrylhydrazyl
-
- EC50
-
- half maximal effective concentration
-
- LB
-
- Luria Bertani
-
- NP
-
- nanoparticle
-
- PBS
-
- phosphate buffer solution
-
- PC
-
- l-α-phosphatidyl choline
-
- TBARS
-
- thiobarbituric acid reactive substances
-
- TCA
-
- trichloroacetic acid
-
- XRD
-
- X-ray diffraction
1 Introduction
During the past 20 years, there has been an upsurge in the development of nanomaterials due to their unique electrical, chemical, optical, thermal, and biomedical properties compared to their bulk analogs 1, 2. Among the numerous applications of these materials in industry, environment, health care, and medical care facilities, much attention has been paid to their antibacterial and anti-biofouling properties. Controlling the microbial population or eliminating it has always been a challenge due to the antibiotic resistance that several bacteria exhibit 3, 4. Potentially pathogenic Gram-negative Escherichia coli and pathogenic Gram-positive Staphylococcus aureus are two common bacteria strains responsible for human bacterial infections. Both bacteria are commonly found in the environment and mostly on human and animal skin. Insufficient elimination of the bacteria combined with their quorum sensing ability results in the formation of biofilms, virtually on every surface like rocks, floors, water pipes, catheters, boat hulls, etc. 4-6. Biofouling – the condition in which a biofilm is formed on surfaces that come into contact with water – annually costs millions of dollars for the removal of the biofilms or replacement of the affected parts 7. It is conceivable, therefore, that the antibacterial and anti-biofouling properties of materials are important considering the growing demand for new, effective, and environmentally friendly antibacterial agents 1, 8.
Benign metal-based nanomaterials have high potential for application in environmental processes, such as water cleaning, disinfection, and removal of biofilms from surfaces, which come into contact with water. So far, different metals such as silver, gold, iron, copper as well as their oxides and composites with TiO2 have been tested, as antibacterial agents 9-14. Silver and gold are well known since ancient times for their biocidal activity against fungi, viruses, and especially against bacteria. This ability was attributed to the oligodynamic effect that these metals exhibit 15, 16. Τhe antibacterial activity of metals, at nanoscale, is substantially higher than that of their bulk analogs 17, 18 since it is related to their surface area, which is definitely higher in the first case 19. Considering that the average bacteria cell size is ∼2 µm, nanomaterials are capable of penetrating the cellular membrane through the pores and ultimately inhibit their growth 20, 21. Plenty of mechanisms have been proposed to clarify the toxic effects of certain nanoparticles (NPs), such as the generation of reactive oxygen species, oxidation, lipid peroxidation, etc. 18, 22, 23. However, it still remains unclear how the antibacterial mechanism of certain nanomaterials comes into operation.
Of the metallic NPs which can be used as antibacterial agents, iron and copper NPs are preferable considering their low cost of synthesis and the convenience they provide to control their shape and size, through their synthetic pathways 21, 24. Metallic Cu NPs have been synthesized and their antimicrobial characteristics have been investigated against E. coli and Bacillus subtilis 25. The NPs are susceptible to oxidation in the presence of air, particularly, once Cu2+ is formed 26. It has been proposed that the mechanism through which the Cu NPs reduce bacteria viability is related to protein inactivation via thiol interaction. In this particular case of Cu NPs, the low redox potential causes surface oxidation, which decreases the antimicrobial characteristics, due to the fact that copper oxide is less reactive than metallic Cu 27. To obtain Cu NPs with high antimicrobial properties, the size control of NPs and the presence of a surface coating to prevent their oxidation are of importance.
Metallic Fe NPs have also shown antimicrobial properties and they can serve as a cost-effective alternative to many of the applications in which silver is being used. However, the applicability of nano Fe0 is limited by its oxidative corrosion 28. It is generally accepted that nano Fe0 has a core-shell structure with a Fe0 core surrounded by an oxide/hydroxide shell, which grows thicker with the progress of iron oxidation 29. Martin et al. 30 determined the oxide layer thickness in core-shell Fe0 NPs by using high resolution transmission electron microscopy and high resolution X-ray photoelectron spectroscopy and the values were in the range of 2–4 nm and 2.3–2.8 nm, respectively. In addition to the antibacterial properties, the antioxidant properties of metal-based NPs have been documented in an attempt to correlate them with reactive oxygen species generation and the oxidative stress 31.
The antibacterial and anti-biofouling properties of metallic Fe, Cu NPs have not yet been documented in detail. This knowledge is important for their use in applications, such as water disinfection, coating of water treatment filters, or even as disease management factors 1, 32. Moreover, bimetallic Fe–Cu NPs could be a reasonable alternative, which combines the above-mentioned advantages of both metallic particles and overcomes the lack of magnetic properties of Cu, in single-metal preparations. Recent innovations in nanomaterial synthesis and production have resulted in substantial cost reductions and increased availability of the metallic NPs for large scale field applications. In this paper, an activity study of Cu, Fe, and Fe–Cu NPs on E. coli and S. aureus was carried out in order to obtain an integrated view of their capabilities with respect to their antibacterial and anti-biofouling behavior. In addition, the inhibition of lipid oxidation following the thiobarbituric acid reactive substances (TBARS) method and the antioxidant activity of the above nanomaterials using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) (free radical scavenging assay) were examined. The three metallic nanomaterials were synthesized using a straightforward wet-chemical method and were properly characterized.
2 Materials and methods
2.1 Reagents
All chemicals used were of analytical grade. Double distilled water (DDW) was used throughout the experiments. (NH4)2Fe(SO4)2 · 6 H2O, CuSO4 · 5 H2O, NaBH4, NaCl, KCl, KH2PO4, ascorbic acid, crystal violet, butanol, FeCl3, and trichloroacetic acid (TCA) were purchased from Merck (Darmstadt, Germany). Bacteriological peptone and yeast extract were from Biolife (Milano, Italy). Absolute ethanol was purchased from Scharlau (Barcelona, Spain), Na2HPO4 was from Riedel-de Haën (Seelze, Germany) and methanol from Fischer Scientific (Loughborough, Leicestershire, UK). 3,5-Di-tert-4-butylhydroxytoluene (BHT) and 1,1-diphenyl-2-picrylhydrazyl were obtained from Aldrich (Steinheim, Germany). NaH2PO4, 2-thiobarbituric acid and l-α-phosphatidyl choline (PC) from soybean were purchased from Fluka Chemie (Buchs SG, Switzerland). Sea water was collected from Thermaic Gulf (salinity between 35.7 and 37.5 33). Phosphate buffer saline (PBS) was made up of 8.0 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4 in 1 L DDW. The pH of the PBS solution was adjusted to 7.4 with 0.1 mol/L HCl. Luria Bertani (LB) liquid medium was made up of 10 g/L NaCl, 5 g/L yeast extract, and 10 g/L bacteriological peptone. The pH of the LB was adjusted to 7.4 with 0.1 mol/L NaOH prior to autoclaving. All solutions and glassware were sterilized by autoclaving at 121°C for 15 min, at 15 psi.
2.2 Apparatus
X-ray diffraction (XRD) analysis was performed on a D8 Advance diffractometer from Bruker AXS (Madison, USA) using CuKα (λ = 1.5406 Å) radiation. The particles' diameters and standard deviations were calculated by applying Scherrer's equation to each peak in the XRD diffractograms. This signifies that the equation was applied two, four, and five times for Fe, Cu, and Fe–Cu NPs, respectively. High resolution-transmission electron microscopy (HR-TEM) images were obtained with a JEOL JEM-2100 microscope operated at 200 kV equipped with LaB6 filament. Nanomaterials were deposited on carbon-coated copper grids after suspending them in DDW. An Autosorb-1 porosimeter from Quantachrom Instruments (Boynton Beach, USA) was used to determine the surface area using nitrogen adsorption–desorption porosimetry, according to the Brunauer–Emmett–Teller (BET) method. Atomic absorption spectrometry (AAS) measurements were carried out on a GBC AAS 932 PLUS atomic absorption spectrometer (GBC Scientific, Victoria, Australia), at 324.7 and 372.0 nm for Cu and Fe, respectively. The molecular absorbance measurements were carried out on a Shimadzu UV-2100 spectrophotometer (Kyoto, Japan) using a quartz cuvette of 10 mm path length.
2.3 Synthesis of nanomaterials
The Fe–Cu bimetallic NPs were prepared by a wet co-precipitation method using sodium borohydride as reducing agent. Stoichiometric quantities (Fe/Cu, 1:1) of 565 mg CuSO4 and 785 mg (NH4)2Fe(SO4)2 · 6 H2O were dissolved in a 100-mL glass beaker containing 20 mL of deaerated DDW. A total of 400 mg NaBH4 was then rapidly added to the clear solution under vigorous stirring. The mixture was further allowed to stir under ambient conditions for 25 min. The magnetic solid was harvested using a hand magnet, rinsed five times with 40 mL DDW and three times with 40 mL acetone. The solid was finally dried in the oven at 60°C, for 30 min. Iron and Cu NPs were prepared in the same way, with the addition of the respective salt in each case so that black precipitates were obtained. For the non-magnetic Cu NPs, the particles were left to precipitate and the supernant was decanted away. All particles were suspended in ethanol to avoid oxidation and conversion into oxide forms. Suspensions of the metal NPs at the appropriate concentrations were prepared in LB medium using ultrasonication to avoid agglomeration.
2.4 Dissolution of nanomaterials in working media
Suspensions of the three nanomaterials were prepared in stirred LB and PBS at concentrations of 5, 50, 100, and 200 µg/mL, at 37°C, under stirring. At time intervals of 0.5, 1, 2, and 4 h from the start of stirring, suspensions were centrifuged and the concentrations of the dissolved Fe and Cu were measured in the supernatants by AAS 34.
2.5 Cultivation of bacteria
Staphylococcus aureus (NCTC 6571) and E. coli DH5 α-strains were stored in glycerol 20% (v/v), at −80°C. Two bacterial pre-inoculums of each bacterium were prepared by adding a small amount of bacterial spores in 10 mL of fresh LB medium and incubating it overnight at 37°C. A culture was prepared by inoculating 2.5 mL of the pre-inoculums in 250 mL LB and by incubating for 16 h at 37°C, in an incubator shaker, at 250 rpm.
2.6 Antibacterial activity of the NPs
The antibacterial activity of the three nanomaterials was evaluated by drawing the growth curves of the two bacterial species incubated with the nanomaterials at concentrations in the range of 5–200 µg/mL 31, 32. Control experiments were performed in the absence of the NPs. The absorbance of a grown bacterial culture was measured at 600 nm and was adjusted to 0.1 (corresponds approximately to 106 colony forming units (CFU) per mL) by dilution with LB containing the well dispersed NPs, at different concentrations. The new culture was placed in an incubator shaker at 250 rpm at 37°C, for 24 h. Meanwhile, the absorbance was measured at intervals of 30 min for the first 3 h and 1 h intervals for the remaining 21 h. The decrease in absorbance during the bacterial growth, compared to the control cultures, corresponds to the inhibition of the growth caused by the NPs.
2.7 Anti-biofouling assay
A grown bacterial culture was diluted with LB in a ratio of 1:100, containing 50% sea water and different concentrations of the three nanomaterials (5, 50, 100, 200, and 1000 µg/mL) 1. After 48 h of incubation at 28°C, the LB medium, containing 50% sea water, was removed and the test tubes were washed with PBS. Thereafter, 100 µL of crystal violet (0.1% w/v) was added and the tubes were incubated for 20 min at 28°C. After removing the crystal violet, the tubes were washed once with PBS. In order to quantify the attached cells, crystal violet was dialyzed in absolute ethanol and the absorbance was measured at 570 nm. The quantity of crystal violet bound by the biofilm is proportional to the number of bacteria and thus to the growth of the biofilm. The reduction of the biofilm was correlated with the cells treated with the nanomaterials.
2.8 Inhibition of lipid oxidation – TBARS assay
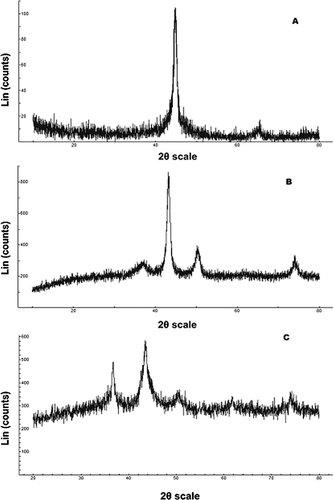
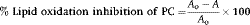 (1)
(1)2.9 DPPH free radical scavenging assay
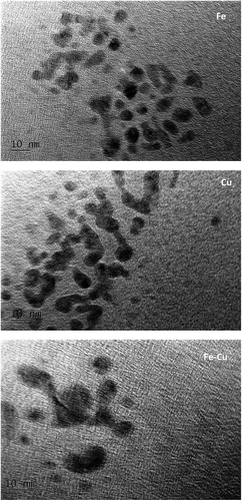
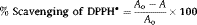 (2)
(2) (3)
(3)3 Results and discussion
3.1 Characterization of the nanomaterials
Figure 1 represents the XRD diffractograms of the synthesized nanomaterials, which match well with the diffraction pattern of the target ones. This is corroborated by the presence of the diffraction peaks (Bragg peaks) at 44.8° and 65.0° for Fe and 43.7°, 50.7°, and 74.3° for Cu NPs, which correspond to specific planes in cubic systems. As for the diffraction angles of the bimetallic nanomaterial, the characteristic peaks lie at 44.5° (broad peak), 50.7°, 65.0°, and 74.3°. The diffraction peaks of Cu NPs are similar to those of pure bulk crystalline copper, in terms of angular positions but they are relatively broad, as the mean size of the particles is of the order of nanometers 36, 37. A characteristic peak at 2θ ∼44.7° in the XRD pattern of Fe NPs indicates its presence, predominantly in the Fe0 state. Reflections due to iron oxides, such as magnetite, γ-Fe2O3 or hematite (2θ ∼36°) are not observed. The broad diffraction peaks observed close to the diffraction angle of 36.4° of Fig. 1 (patterns B and C) can be attributed to cuprite and cuprite/iron oxides, respectively. This denotes the presence of thin copper oxide and/or iron oxide passivation layers on the surface of Cu and Fe–Cu NPs, which prevent further oxidation of Cu and Fe atoms. Interestingly, the magnetic Fe and Fe–Cu NPs, collected after an antibacterial activity experiment carried out for 24 h, kept their original black color while their XRD patterns, before and after this treatment, were alike. This evidence points toward stability of the materials, at least for a period of 24 h.
The mean particle diameter of the nanomaterials, as calculated from the XRD patterns using the Scherrer equation, showed the following results: Fe, 6.6 ± 0.9 nm; Cu, 5.3 ± 0.8 nm, and Fe–Cu, 15 ± 3 nm. HR-TEM images (Fig. 2) verify the presence of few nanometer-sized nanoparticles (black spots) produced by the wet-chemical method with diameters close to those calculated by the Scherrer's equation. Finally, the BET surface areas of the materials are 78, 73, and 81 m2/g for Fe, Cu, and Fe–Cu, respectively.
LB and PBS were selected as media to study the dissolution of metals in suspensions of nanomaterials. The former is the medium of the antibacterial study and the latter approximates to the sea water composition for the anti-biofouling study. It was found that after 4 h of stirring, the dissolved Cu and Fe in the supernatant of LB were non-detectable. The concentrations of the respective metals in PBS supernatant were as low as between 0.1 and 0.8 µg/mL, when the nanomaterial content laid in the range of 5–200 µg/mL. This rationalizes the fact that NPs are not susceptible to oxidation in the presence of air since Cu and Fe ions barely form under these conditions 26.
3.2 Antibacterial activity
The mean absorbance values of three experimental measurements for the two bacterial cultures at different suspensions of the nanomaterials are depicted in Fig. 3. All the materials tested in both bacterial strains have similar pattern of action, with E. coli being more vulnerable than S. aureus in the presence of NPs. Moreover, increased concentrations of the materials led to faster growth inhibition of the bacteria. In all cases, though, NPs concentration of 200 µg/mL was able to totally inhibit the growth of bacteria. As regards the growth inhibition of bacteria at the lowest tested concentration of nanomaterials, Cu inhibited growth by 50% after 4 h of incubation while 5 and 6 h are needed for Fe and Fe–Cu, respectively, to achieve the same growth inhibition under the same conditions. Of the three materials tested, Cu had a noticeable antibacterial effect on E. coli, at all concentrations tested, while Fe was the least effective of all – roughly 75% less effective than Cu at the same concentrations. A concentration of Cu NPs as low as 5 µg/mL was able to reduce to half the maximum absorbance of E.coli bacterial culture while concentrations of 100 and 200 µg/mL were able to totally inhibit the growth of bacteria. When the concentrations of the materials were the lowest (i.e., 5 µg/mL), the antibacterial activity of Fe–Cu was slightly better than that of Fe while at highest concentrations, Fe was proved to be more effective. Definitely, Cu seems to be superior as antibacterial material against E. coli.
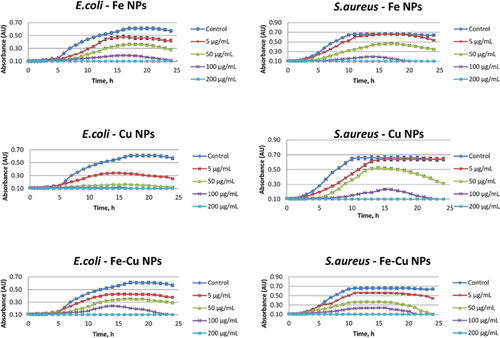
Staphylococcus aureus was more resistive to the studied materials than E. coli. Iron and Cu NPs exhibited almost the same inhibition against S. aureus at all concentrations tested whereas the antibacterial activity of Fe–Cu against E. coli was higher by ∼25% than the antibacterial activity against S. aureus, at the lowest concentration tested.
The as-prepared materials with inherent antibacterial properties may have multiple activity mechanisms, such as release of antibacterial metal ions from the particle surface and cell wall penetration or membrane damage because of the physical properties of NPs. Released ions are also able to enter the membrane of the bacteria and react with amino acids, DNA or other intracellular metabolites, ceasing the metabolomic pathways that these molecules are part of 27. In the studied case, the concentrations of metal ions released in the incubation medium were non-detectable as mentioned above. To pinpoint how the free ions of Cu(II) and Fe(III) may affect the antibacterial activity, relevant experiments were conducted using solutions of increasing concentrations of Cu(II) and Fe(III) up to 10 µg/mL. It was found that the ions had no antibacterial activity at the concentrations tested and therefore, the observed antibacterial activity of these nanomaterials is not associated with the release of metal ions. This reinforces the idea that the presence of intact NPs themselves is of great importance to antibacterial activity while the size and shape of nanomaterials are two determining factors that can affect the effectiveness since the different forms (e.g., needles, grains, etc.) interact with the membrane in a different way 21.
The cell wall differences between the bacteria may be another reason to cause variations to the mode of action of the materials, thus resulting in different susceptibility to them. The Gram-positive bacteria S. aureus were more tolerant of the NPs tested than Gram-negative E. coli, as already demonstrated above. The presence of the thick peptidoglycan layer in Gram-positive species can provide an additional structural barrier to harmful elements of the environment. However, direct contact of NPs with the cell wall, even with the peptidoglycan layer, can cause holes resulting in mortality of the cells, as reported for Cu 26. Lee et al. 28 concluded that E. coli inactivation in the presence of Fe0 NPs might be mediated by the penetration of NPs with an average dimension of 10–80 nm through the cell wall, followed by their interaction with the intracellular oxygen. This results in oxidative stress and eventually bacterial cell death.
3.3 Anti-biofouling assay
The biofilm inhibition study was carried out in triplicate and the mean values of inhibition are depicted in Fig. 4. Although E. coli proved to be more prone than S. aureus to the antibacterial properties of the materials tested, the biofilm formed by E. coli was less susceptible to the effect of the NPs as compared to that formed from S. aureus. It is somewhat counter-intuitive and surprising that a Gram-positive species is more vulnerable than Gram-negative species to the anti-biofouling properties of metallic NPs. Apparently, the mechanism of biofilm inhibition depends on direct contact of NPs with the attached cells. Since the bacterial cells are attached to the tube walls and they are not dispersed in a solution, the interaction between the materials and the cells is more difficult; therefore, the activity of the materials lessens 8.
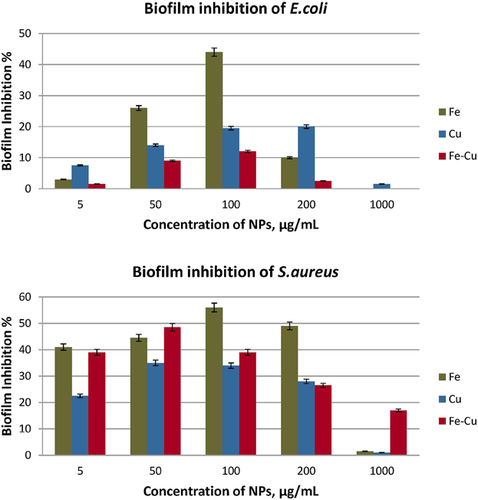
With respect to the nanomaterials studied, highest anti-biofouling activity was attained at a concentration of 100 µg/mL. Thereafter, the effectiveness deteriorated due, probably, to the tendency of NPs to aggregate, thus resulting in lower bioavailability. In the case of E. coli, 44% inhibition was achieved in the presence of Fe NPs, followed by 20% inhibition from Cu NPs and finally 12% inhibition from Fe–Cu, when the material concentration was 100 µg/mL. Likewise, maximum inhibition of 56% was achieved on S. aureus after treatment with Fe at a concentration of 100 µg/mL whereas biofilm growth was inhibited by 35 and 49% with Cu and Fe–Cu, respectively, at 50 µg/mL. Although Cu was the most effective among the three materials tested, in terms of bacterial growth inhibition, Fe exerted twice as high biofilm inhibition on E. coli as Cu. Likewise, the anti-biofouling activity of Fe was approximately 1.5 times higher than that of Cu on S. aureus. The inhibition of the biofilm formation recorded for Fe-Cu NPs was significantly low on E. coli falling between the inhibition values for Fe and Cu on S. aureus. Finally, it is noteworthy that the bimetallic Fe–Cu nanomaterial is a significant biofilm inhibitor for S. aureus, at concentrations <100 µg/mL (e.g., 50 µg/mL) implying its applicability as an alternative magnetic material for such purposes.
3.4 TBARS assay
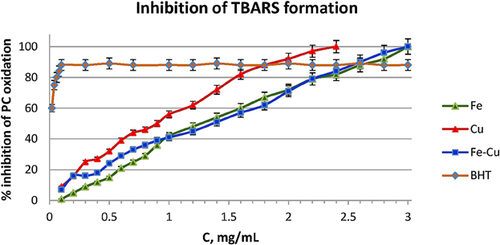
The inhibition of TBARS formation expresses the protection against lipid peroxidation. The concentrations examined varied from 0.1 to 3.0 mg/mL for each nanomaterial. Triplicate measurements of the inhibition of lipids peroxidation proved to be dose dependent in all three cases (Fig. 5), albeit considerably lower than that of the standard antioxidant (i.e., the BHT), at low concentrations. For Fe and Fe–Cu NPs, 100% inhibition was achieved with 3 mg/mL while for Cu NPs the same effect could be achieved with 2.5 mg/mL, signifying that Cu is more active on inhibition of lipids oxidation. As the NPs suspensions in the antibacterial and anti-biofouling study was limited up to a concentration of 200 µg/mL, it appeared that the lipid peroxidation was hardly inhibited by the protective effects of the materials. Hence, it is most likely that the antibacterial mechanism passes through the damage or collapse of bacterial lipid membrane.
3.5 DPPH free radical scavenging assay
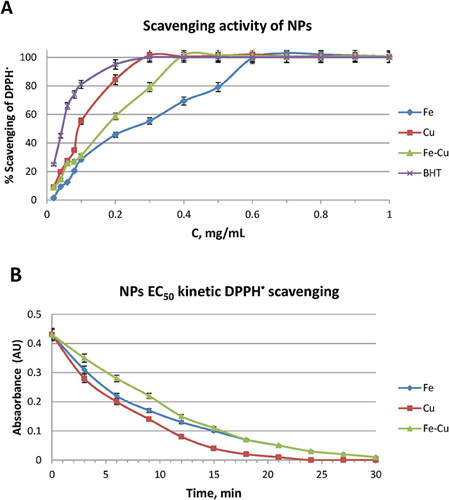
Free radical-related processes were found to be responsible for a variety of oxidative damages leading to adverse health effects and diseases in the environment. Figure 6A shows the DPPH• scavenging activity values from triplicate measurements as a function of concentration of NPs, which increases in a dose-dependent manner. All three materials were capable of effecting 100% scavenging in the DPPH•, at different concentrations and were compared with a standard antioxidant (i.e., BHT). Best performance was attained using material suspensions at concentrations of 0.61, 0.29, and 0.39 mg/mL for Fe, Cu, and Fe–Cu, respectively. According to the EC50 values, the scavenging properties of the nanomaterials, in increasing order, are: Fe (0.30 mg/mL) < Fe–Cu (0.19 mg/mL) < Cu (0.14 mg/mL). Evidently, Cu, Fe, and Cu–Fe NPs have the capability to transfer their electron densities toward the free radical located at the nitrogen atom of DPPH•. Iron with its relative low standard electrode potential (E0, −0.41 V) and also Cu can be effective and environmentally friendly electron donors. Taking into account that the free radicals are short-living species – especially in biological systems – the time needed for a compound to scavenge radicals is a critical factor, too. Kinetic experiments were carried out to examine the antioxidant activity as a function of time, using the EC50 concentrations. From the curves of Fig. 6B, the TEC50 values were calculated and the respective AE values derived for the materials under scrutiny. If the evaluation criterion is the AE value – which takes into account the time needed for 50% reduction of the initial absorbance using the EC50 concentration of the material – the scavenging activity, in increasing order is: Fe (0.55) ∼ Fe–Cu (0.58) < Cu (1.19). It is obvious that Cu NPs is more effective as radical scavenger, in every way.
4 Concluding remarks
In this work, the antibacterial, anti-biofouling, and antioxidant properties of three metallic, easily synthesized benign nanomaterials were studied. All of them proved to affect E. coli and S. aureus, since small amounts can totally inhibit the growth of the bacteria and their biofilm formation, compared to control cultures. Their action is attributed to the nature and the size of the materials, rather than the presence of the respective ions, considering their insignificant dissolution. However, the exact mechanism of action of each material is not yet clearly determined. A metabolomic study is underway in our laboratory to provide data, which will make it easier to account for the differences observed.
Besides, the antibacterial properties, the materials exhibited concentration- and time-dependent free-radical antioxidant activity with relatively small amounts of the materials while lipid peroxidation inhibition is attained at high concentrations. Since Fe and Cu are natural resources in abundance and the synthesis of the respective nanomaterials is fast and straightforward, their use especially in the environment is a cost-effective alternative to the present technologies. What is more, the unique surface reactivity of nanoscale dimensions along with the magnetic properties of Fe and Fe–Cu NPs make them good candidates for treating bacteria in environmental applications.
The authors have declared no conflicts of interest.




