Hybrid Homogeneous and Heterogeneous Photocatalytic Processes for Removal of Triphenylmethane Dyes: Artificial Neural Network Modeling
Abstract
Removal of two triphenylmethane dyes, Acid Fuchsin (AF) and Malachite green (MG), was studied by hybrid advanced oxidation processes of homogeneous (UV/Fe2+/H2O2) and heterogeneous (UV/TiO2−SiO2) photocatalysis. A comparison of various processes for removal of model pollutants was performed. The results showed that the utilizing hybrid photocatalytic processes in the presence of silica leads to rapid removal of pollutants, which may be ascribable to the synergistic influence of produced various radical species. The effects of operational variables were studied on the efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process. An artificial neural network (ANN) model was intended to predict the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process under different operational conditions. The results indicated that there is a good concurrence between the ANN predicted values and experimental results with a correlation coefficient of 0.9873 and 0.9774 for removal of AF and MG dyes, respectively. The designed neural network model gives a dependable technique for modeling the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process. Moreover, the relative significance of each variable was computed based on the input-hidden and hidden-output connection weights of the neural network model. The initial concentration of dyes was the most significant variable in the removal efficiency.
Abbreviations
-
- AF
-
- Acid Fuchsin
-
- AOP
-
- advanced oxidation process
-
- ANN
-
- artificial neural network
-
- BET
-
- Brunauer–Emmett–Teller
-
- EDX
-
- energy dispersive X-ray
-
- MG
-
- Malachite green
-
- MSE
-
- mean square error
-
- TEM
-
- transmission electron microscopy
-
- XRD
-
- X-ray diffraction
1 Introduction
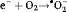 (1)
(1)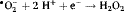 (2)
(2)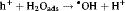 (3)
(3)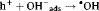 (4)
(4)The hydroxyl radicals are very powerful oxidants and known to be responsible for the degradation of organic pollutants 10.
A combination of both adsorption and heterogeneous photocatalysis into a single process could offer an alternative method in wastewater treatment 11. Silica is widely used as adsorbent with excellent adsorption capacity due to their large specific surface area and porous nature. TiO2–SiO2 catalyst has been considered an advanced material to replace pure TiO2, due to its different surface chemical properties, high catalytic activity, and low cost 12, 13. High adsorption capacity of SiO2 improves the photocatalytic efficiency of TiO2 by increasing the concentration of the target pollutant near the TiO2 sites relative to the solution concentration of a pollutant. In the photocatalytic processes produced oxidizing species such as hydroxyl radicals does not migrate very far from the active centers of the TiO2, thus the pollutant removal process mainly happens on the surface of TiO2. Silica provides a synergistic effect by creating a common interface between the SiO2 and TiO2 particles 13-15.
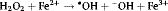 (5)
(5)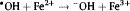 (6)
(6)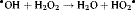 (7)
(7)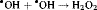 (8)
(8)The removal efficiency of the Fenton process can be accelerated by irradiation with UV light (photo-Fenton process) 18. The effect of UV light is the creation of extra •OH radical species, as well as to regenerate Fe2+ catalyst from the photoreduction of Fe3+. The reduction of Fe3+ back to Fe2+ is critical for the progress of the Fenton process 19.
A literature review revealed that the combination of photo-Fenton and photocatalytic systems in the presence of silica for removal of organic pollutants was not investigated in detail. The aim of this study was utilizing hybrid homogeneous and heterogeneous photocatalytic processes in the presence of silica, to obtaining rapid removal of organic pollutants. In order to achieve the goal of creating an adsorbent-photocatalyst system, TiO2–SiO2 catalyst was prepared via the impregnation method and characterized by various techniques. Because of the chemical structure of the organic dyes which has a considerable effect on its removal rate 20, in the present work removal of two triphenylmethane dyes with different molecular structures, Acid Fuchsin (AF) as an anionic dye and Malachite green (MG) as a cationic dye, were studied by using individual and hybrid removal processes. A neural network model was developed to estimate the effect of operational variables on the removal efficiency of UV/Fe2+/H2O2/TiO2–SiO2 hybrid process. Recently, ANNs approach has been employed in various engineering and scientific applications. Because of the complexity of the reactions involved in the heterogeneous photocatalytic processes, application of ANN as a performance prediction tool is a useful technique 21.
2 Materials and methods
2.1 Materials
All chemicals were utilized as received without further purification. TiO2 nanoparticles (Merck) having Brunauer–Emmett–Teller (BET) surface area 10 m2 g−1 and pure anatase phase. The silica used for this study was highly ordered mesoporous LUS-1 with BET surface area 900 m2 g−1. The preparation of silica LUS-1 has been detailed in a previous report 22. Hydrogen peroxide solution (30%), 6 M sodium hydroxide (NaOH), 0.1 M sulfuric acid (H2SO4), ferrous sulfate heptahydrate (FeSO4 · 7 H2O), and triphenylmethane dyes (AF and MG) were obtained from Merck. The molecular structures of AF and MG dyes are given in Supporting Information Fig. S1.
2.2 Preparation of TiO2 impregnated SiO2 photocatalyst
The preparation of TiO2 nanoparticles supported on SiO2 was performed via impregnation method 23. First, a powder mixture of 1 g SiO2 and TiO2, 50:50 w/w, was ground completely in an agate mortar. Then, the mixed powder was added to 100 mL boiling deionized water and dispersed for 15 min utilizing a probe sonicator (Bandelin HD 3200, 200 W). The suspension was stirred for 24 h and thereafter dried in an air oven at 80°C for 12 h. Then the dried solids were calcined at 450°C for 1 h.
2.3 Characterization of TiO2 impregnated SiO2 photocatalyst
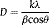 (9)
(9)2.4 Hybrid photo-Fenton and photocatalytic experiment
Hybrid photo-Fenton and photocatalytic removal experiments were performed in a batch quartz reactor. A 15 W (UV-C) mercury lamp (Philips, Holland, 254 nm), situated on top of the reactor was used as the light source. In each test, desired amount (0.1–0.6 g L−1) of the TiO2-impregnated SiO2 catalyst was dispersed in 100 mL deionized water for 15 min using ultrasound irradiation to enhance the dispersion of particles in water 25. Then the prepared catalyst suspension and desired concentration of dye (5–30 mg L−1), and Fenton reagents (H2O2 (0.2–0.7 g L−1) and Fe2+ (0.05–0.3 mmol L−1)) were transferred into the reactor. The process was carried out at pH 3.0 ± 0.1, adjusted by the addition of NaOH or H2SO4. Iron in its Fe2+ state acts as a photocatalyst in the Fenton process and requires a working pH < 4 26. The photocatalytic removal process was started by turning on the UV lamp and stirring with a magnetic bar to ensure proper transport of the reactants. After an irradiation time of 25 min, 5 mL samples were taken out from the reactor, centrifuged (Sigma 2-16P), and then the concentration of the dyes was estimated by UV-Vis spectrophotometry (Rayleigh UV-1600) using the maximum wavelength (λmax) of the dyes (545 nm for AF and 617 nm for MG).
2.5 ANN modeling
All neural network analysis was done using Matlab software, 2011a version, according to the method described in a previous work 9. A three-layer neural network with a sigmoidal transfer function with back-propagation algorithm was designed in this study.
3 Results and discussion
3.1 Characterization results
Figure 1 depicts the XRD pattern of TiO2 nanoparticles supported on silica. All XRD reflections can be indexed to the pure anatase phase of TiO2 (JCPDS no. 21-1272). No rutile phase XRD reflections were detected for the TiO2 particles. The mean crystallite size of TiO2 particles supported on silica was calculated by using Eq. 9, from the (101) reflection of anatase at 2θ = 25.28° and it was found to be about 47 nm.

The TEM image of the nanosized TiO2-impregnated SiO2 photocatalyst is demonstrated in Fig. 2a. From the TEM image, the mean particle size of the TiO2-impregnated SiO2 photocatalyst is estimated to be about 50 nm. This result is in agreement with the mean particle size estimated from the XRD pattern. The composition of the TiO2-impregnated SiO2 photocatalyst was studied with EDX analysis. The EDX results (Fig. 2b) obviously affirm the presence of Si, Ti, and O elements. Also, the results revealed that the weight ratio of Ti to Si is about 1:1 for the TiO2-impregnated SiO2 sample.
3.2 Comparison of various removal processes
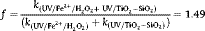 (10)
(10)
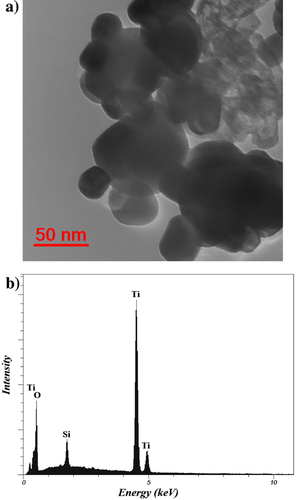
Thus, a synergistic effect is observed for the hybrid system.
3.3 Effects of operational variables on removal efficiency
To study the efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid processes in the removal of AF and MG dyes under various conditions, effects of six operational parameters including TiO2−SiO2 dosage, initial dye concentration, initial Fe2+ concentration, initial H2O2 concentration, initial pH of the solution, and UV-light intensity, were investigated.
3.3.1 The effect of initial dye concentration
To study the influence of the initial dye concentration on the removal rate of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process, the dye concentration varied from 5 to 30 mg L−1, whereas the other variables were kept constant. According to the results given in Tab. 1, the removal rate of both AF and MG dyes decreased as the initial dye concentration increased. An increase in the concentration of dyes from 5 to 30 mg L−1 decreased the removal rate of both AF and MG dyes from 71 and 97% to 17 and 50%, respectively. The higher removal efficiency of MG than AF could be because of the positively charged structure of MG molecules that interacted with the photogenerated electrons of TiO2−SiO2 catalyst and this prompts the quick oxidation of MG by free holes 10. AF molecules with negatively charged sulfonic groups (–SO3−) repel the photogenerated electrons of the TiO2−SiO2 catalyst. This phenomenon enhances the probability of charge-carrier recombination and prompts the end of the photocatalytic oxidation 10. With increasing the initial dye concentration, more dye molecules are adsorbed on the TiO2−SiO2 surface, and consequently the chance of forming reactive species such as •OH radicals will be diminished 23, 32, 33. On the other hand, with increases in the initial concentration, the dye solution gets impermeable to UV irradiation because the molar extinction coefficient of the dyes at 254 nm (the wavelength of UV lamp) is very high. In that case, the UV photons get intercepted before reaching the photocatalysts surface 23, 34, 35.
| Removal rate (%) | |||||||
|---|---|---|---|---|---|---|---|
| Initial dye concentration (mg L−1) | TiO2−SiO2 dosage (g L−1) | Initial Fe2+ concentration (mmol L−1) | Initial H2O2 concentration (g L−1) | Initial solution pH | UV light intensity (W m−2) | AF | MG |
| 5 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 71 | 97 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 59 | 89 |
| 15 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 48 | 80 |
| 20 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 40 | 67 |
| 25 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 29 | 56 |
| 30 | 0.4 | 0.05 | 0.4 | 3 | 56.5 | 17 | 50 |
| 10 | 0.1 | 0.05 | 0.4 | 3 | 56.5 | 19 | 42 |
| 10 | 0.2 | 0.05 | 0.4 | 3 | 56.5 | 38 | 61 |
| 10 | 0.3 | 0.05 | 0.4 | 3 | 56.5 | 50 | 78 |
| 10 | 0.5 | 0.05 | 0.4 | 3 | 56.5 | 58 | 85 |
| 10 | 0.6 | 0.05 | 0.4 | 3 | 56.5 | 55 | 82 |
| 10 | 0.4 | 0.1 | 0.4 | 3 | 56.5 | 64 | 92 |
| 10 | 0.4 | 0.15 | 0.4 | 3 | 56.5 | 69 | 93 |
| 10 | 0.4 | 0.2 | 0.4 | 3 | 56.5 | 66 | 95 |
| 10 | 0.4 | 0.25 | 0.4 | 3 | 56.5 | 65 | 91 |
| 10 | 0.4 | 0.3 | 0.4 | 3 | 56.5 | 59 | 85 |
| 10 | 0.4 | 0.05 | 0.2 | 3 | 56.5 | 41 | 63 |
| 10 | 0.4 | 0.05 | 0.3 | 3 | 56.5 | 48 | 73 |
| 10 | 0.4 | 0.05 | 0.5 | 3 | 56.5 | 65 | 93 |
| 10 | 0.4 | 0.05 | 0.6 | 3 | 56.5 | 61 | 91 |
| 10 | 0.4 | 0.05 | 0.7 | 3 | 56.5 | 57 | 85 |
| 10 | 0.4 | 0.05 | 0.4 | 5.1 | 56.5 | 55 | 85 |
| 10 | 0.4 | 0.05 | 0.4 | 6.3 | 56.5 | 51 | 82 |
| 10 | 0.4 | 0.05 | 0.4 | 7.6 | 56.5 | 30 | 76 |
| 10 | 0.4 | 0.05 | 0.4 | 8.4 | 56.5 | 21 | 69 |
| 10 | 0.4 | 0.05 | 0.4 | 9.2 | 56.5 | 11 | 67 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 48.5 | 50 | 82 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 43 | 43 | 71 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 36 | 32 | 60 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 29.5 | 25 | 54 |
| 10 | 0.4 | 0.05 | 0.4 | 3 | 24 | 21 | 49 |
3.3.2 The effect of TiO2−SiO2 dosage
To study the effect of TiO2−SiO2 dosage on the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process, the TiO2−SiO2 dosage varied from 0.1 to 0.6 g L−1, whereas the other variables were kept constant. Tab. 1 shows the variation of the removal rate for AF and MG dyes as a function of the TiO2−SiO2 dosage. According to the results given in Tab. 1, the increase in the TiO2−SiO2 dosage until about 0.4 g L−1 enhanced the removal rate of both AF and MG dyes. By varying the TiO2−SiO2 dosage from 0.1 to 0.4 g L−1, the removal rate of AF and MG dyes improved from 19 and 42% to 59 and 89%, respectively. This can be due to an increase in the number of accessible catalytic and adsorption sites on the TiO2−SiO2 surface 23, 36. An improvement on the removal efficiency of the hybrid process with an increase in the TiO2−SiO2 dosage is not remarkable for concentrations of >0.4 g L−1. High turbidity of the solution caused by high catalyst loading leads to a reduction in UV light penetration into the solution 23, 37, 38. A further increase in the TiO2−SiO2 dosage from 0.4 to 0.6 g L−1 decreased the removal rate of AF and MG dyes from 59 and 89% to 55 and 82%, respectively.
3.3.3 The effect of initial Fe2+ concentration
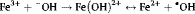 (11)
(11)3.3.4 The effect of initial H2O2 concentration
To study the relationship between the initial H2O2 concentration and removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process, the initial H2O2 concentration varied from 0.2 to 0.7 g L−1, while the other variables were kept constant. Table 1 indicates the influence of the initial concentration of H2O2 on the removal rate of AF and MG dyes. The results indicated that the removal rate of both AF and MG dyes increased as the initial H2O2 concentration increased. An increase in the initial concentration of H2O2 from 0.2 to 0.5 g L−1 increased the removal rate of both AF and MG dyes from 41 and 63% to 65 and 93%, respectively. With increasing the H2O2 concentration, additionally hydroxyl radicals are produced. An improvement on the removal efficiency of the hybrid process with an increase in the H2O2 concentration is not remarkable for concentration of >0.5 g L−1. An increase in the initial concentration of H2O2 from 0.5 to 0.7 g L−1 decreased the removal rate of AF and MG dyes from 65 and 93% to 57 and 85%, respectively. This could be because of the recombination and scavenging of •OH radicals by hydrogen peroxide, according to Eqs. 7 and 8 19, 41-43.
3.3.5 The effect of initial solution pH
The initial solution pH is an important parameter in Fenton reactions for maintaining the effectiveness of the process because the pH value affects the •OH radical production and accordingly the performance of the Fenton process 19. The effect of the initial solution pH on the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process was studied for pH values between 3 and 9.2, while the other variables were kept constant. Table 1 shows the variation of the removal rate for AF and MG dyes as a function of the solution pH. It can be seen from Tab. 1 that the increase in the initial pH decreased the removal rate of both AF and MG dyes. These observations are in agreement with the results of previous studies 44-46, since the maximum hydroxyl radical production is expected from Fenton's reaction around pH 2.8. An increase in the initial pH from 3 to 9.2 decreased the removal rate of AF and MG dyes from 59 and 89% to 11 and 67%, respectively.
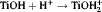 (12)
(12)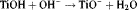 (13)
(13)Under acidic conditions, the adsorption of anionic molecules mainly occurs on the surface of the catalyst because the TiO2−SiO2 surface is positively charged by adsorption of H+. The increase in the removal rate at acidic pH may be because of the high adsorption rate of anionic molecules on the TiO2−SiO2 surface. Unlike acidic conditions, under alkaline conditions, adsorption of cationic molecules mainly occurs on the surface of the catalyst. The increase in the removal rate at alkaline pH is probably related to the high rate of cationic molecules adsorption on the TiO2−SiO2 surface. Under acidic conditions maximum hydroxyl radicals produced from Fenton's reaction and high adsorption rate of AF molecules occurred on the catalyst surface, thus hydroxyl radical production is along with adsorption of AF molecules. While in the case of the MG dye, hydroxyl radical production is not along with the adsorption of MG molecules. As a result, we are faced with a drastic reduction in the removal rate of AF dye with an increase in pH.
3.3.6 The effect of light intensity
To study the influence of UV light intensity on the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process, UV light intensity was varied in the range of 24–56.5 W m−2, while the other variables were kept constant. Table 1 shows the variation of the removal rate for AF and MG dyes as a function of UV light intensity and solution. According to the results given in Tab. 1, decreasing the UV light intensity increased the removal rate of both AF and MG dyes. A decrease in the light intensity from 56.5 to 24 W m−2 decreased the removal rate of AF and MG dyes from 59 and 89% to 21 and 49%, respectively. In the photo-Fenton process, UV light is used for photoreduction of Fe3+ to Fe2+ and photolysis of H2O2. The photolysis rate of H2O2 directly depends on the UV light intensity 19. Therefore, improvement of the removal rate at high UV light intensity is because of the increase in generation of highly reactive •OH radicals from the photo-dissociation of hydrogen peroxide 19, 50. On the other hand, in the photocatalytic process, UV light irradiation provides the photons required for the generation of electron–hole pairs. In the presence of low intensity of UV light, the electron–hole separation rivals with recombination and reduces the generation of •OH radicals 10, 51. When more radiation falls on the TiO2−SiO2 surface (at high light intensity), more hydroxyl radicals are produced and consequently the removal rate increased 52.
3.4 ANN modeling
The effects of operational variables on the removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process were analyzed by utilizing a multilayer feed-forward ANN model. Operational variables (including TiO2−SiO2 dosage, initial dye concentration, initial Fe2+ concentration, UV-light intensity, initial H2O2 concentration, and initial pH of the solution) were chosen as the input of the network and removal efficiency (%) of dyes was chosen as an output of the network.
 (14)
(14)The neuron number of a hidden layer influences the efficiency of the neural network model. In order to assess the best configuration of hidden nodes, experiments were performed by altering the number of neurons from 1 to 20. To avoid any accidental correlation due to the random initialization of the weights, each topology was evaluated three times. Figure 4 demonstrates the correlation between the neuron number in the hidden layer and the mean square error (MSE). The lowest MSE is resulted when 14 neurons were used. Hence, 14 neurons were chosen as the best number of neurons.
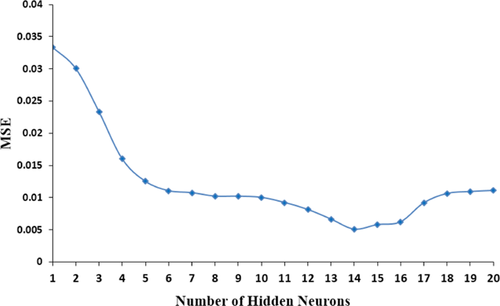
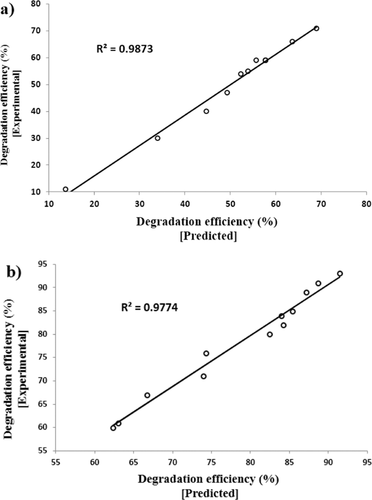
Figure 5 demonstrates a correlation between experimental removal efficiency (%) results and ANN-predicted values for the test set. The correlation coefficients (R2) of the lines are 0.9873 and 0.9774 for the removal of AF and MG dyes, respectively. The R2-value infers an agreeable representation of the hybrid removal process by the model. According to Fig. 5, there is an acceptable concurrence between the ANN-predicted values and the experimental results. Therefore, the designed ANN model is able to predict adequately the removal rate of both anionic and cationic dyes for the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process.
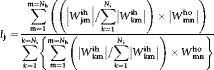 (15)
(15)According to the results given in Tab. 1, among the operational variables, the initial dye concentration has greater influence on the removal rate of both AF and MG dyes. While the initial solution pH was identified as the parameter with great effect on the removal of AF, it was identified as the fourth efficient parameter on the removal of MG. As can be seen in Tab. 2, the initial concentrations of Fe2+ and H2O2 were identified as the parameters with weak effect on the removal of both AF and MG. Nevertheless, the difference in the relative significance of the operation variables is not that significant. The high sensitivity of the network response reveals low flexibility in the process control and the necessity of exact control of all operational parameters at desired values.
| Relative importance (%) | ||
|---|---|---|
| Variable | AF | MG |
| Initial dye concentration | 24 | 20 |
| TiO2−SiO2 dosage | 15 | 17 |
| Initial Fe2+ concentration | 4 | 6 |
| Initial H2O2 concentration | 8 | 11 |
| Irradiation time | 13 | 15 |
| Initial solution pH | 20 | 12 |
| UV light intensity | 16 | 19 |
4 Concluding remarks
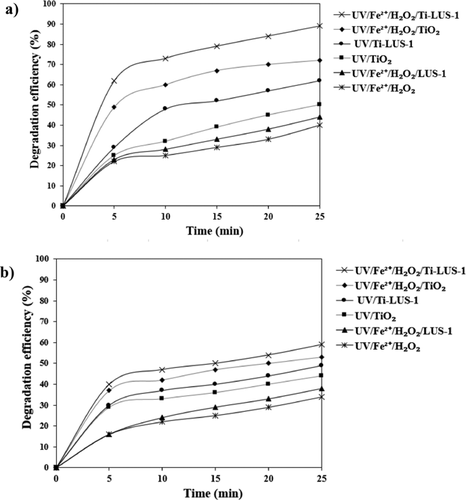
The removal of AF and MG from aqueous solutions has been studied by hybrid and individual homogeneous and heterogeneous photocatalytic processes using titania-impregnated silica. The removal efficiency of different processes was found to be in the order of: UV/Fe2+/H2O2/TiO2−SiO2 > UV/Fe2+/H2O2/TiO2 > UV/TiO2−SiO2 > UV/TiO2 > UV/Fe2+/H2O2/SiO2 > UV/Fe2+/H2O2. The best performance of the UV/Fe2+/H2O2/TiO2−SiO2 hybrid process was observed for operational conditions with optimal values: TiO2−SiO2 dosage of 0.4 g L−1, dye concentration of 5 mg L−1, initial Fe2+ concentration of 0.15−0.2 mmol L−1, H2O2 concentration of 0.4 g L−1, irradiation time of 25 min, initial solution pH of 3, and UV-light intensity of 56.5 W m−2. The removal efficiency of the UV/Fe2+/H2O2/TiO2−SiO2 process under different operational conditions was successfully predicted by utilizing a three-layered back-propagation neural network with 14 neurons in the hidden layer. There was an acceptable concurrence between the ANN-predicted values and the experimental results with an R2 of 0.9873 and 0.9774 for removal of AF and MG dyes, respectively.
Acknowledgment
The authors thank University of Tehran for the support of this work.
The authors have declared no conflicts of interest.




