Separation and Preconcentration of Aluminum by Candida albicans Immobilized on Cellulose Acetate Biomass
Abstract
In the present study, separation parameters of aluminum ions from aqueous solutions by Candida albicans immobilized on the cellulose acetate biomass (CAICAB) were investigated. The optimum experimental conditions for the preconcentration of aluminum were investigated. Amount of CAICAB, effect of common matrix ions and capacity of CAICAB were also obtained. 5 mL of 2 mol L−1 HCl were used for elution of adsorbed aluminum ions on CAICAB. After elution, analyte ions were determined by high resolution continuum source flame atomic absorption spectrometry. Under the optimized conditions, limit of detections for aluminum and adsorption capacity of CAICAB were 0.12 and 327.5 µg g−1, respectively. The method developed was applied to the separation and determination of aluminum in water samples.
Abbreviations
-
- CAICAB
-
- cellulose acetate biomass
-
- HR CS-FAAS
-
- high resolution continuum source flame atomic absorption spectrometry
-
- LOD
-
- limit of detection
-
- PF
-
- preconcentration factor
1 Introduction
Inorganic and organic environmental pollution due to developments in technology is one of the most significant problems of this century. Toxic metals such as Al, Pb, Cr, Ni, Cu Cd, As, and Hg in the environment at low concentrations, especially in drinking water can cause serious health effects. Therefore, heavy metals have been recognized as one of the major problems for human health 1, 2. Aluminum is one of these metals and it has no known essential role in living organisms. Al has been suggested to play a role in the degeneration of nerve cells in brains of humans and experimental animals 3. Therefore, the determination and elimination of Al ions from water and wastewater by using separation and removal techniques are important to protect public health.
Solid-phase extraction is an efficient, sensitive and inexpensive technique to perform removal and separation of metal ions from environmental samples 4-6. Conventionally, several adsorbents including polymeric materials, some species of activated carbon, metal oxide and carbon based nanomaterials, modified silica and biomass have been used as solid phase supporting material for trace metal removing and preconcentration 7, 8.
Preconcentration and removing of metals from water samples using biomass have been much explored in recent years. Biomass of bacteria, algae, and fungi have been used successfully as biosorbents due to low cost, larger surface area, stability against acidic media, and selectivity for some analytes 9. The cell walls of microorganisms have negative charged groups and various functional groups to capture selectively metal ions 10.
The aim of this study was to develop a simple, low cost and environmental-friendly separation and preconcentration method for trace Al in water samples. Different species of microorganism biomasses have been used for the removal and preconcentration of heavy metals from aqueous solutions 11, 12. Candida albicans is an inexpensive and easily cultivable biosorbent for the removal of heavy metal ions from solutions 13. The cell wall surface of C. albicans has various functional groups for the sorption of metal ions. Therefore, C. albicans immobilized on the cellulose acetate biomass (CAICAB) was used as an adsorbent in column. Several analytical parameters were investigated and optimized. The method was applied for separation and determination of aluminum in water samples.
2 Materials and methods
2.1 Apparatus
The analysis was performed by high resolution continuum source flame atomic absorption spectrometry (HR CS-FAAS) (ContrAA 300, GLE, Germany) equipped with a 50 mm burner head and an injection module (SFS-6). All absorption lines of an element in the spectral range of 185–900 nm can be analytically evaluated by using a Xe short-arc lamp as a continuum lamp source. All pH measurements were done with a pH meter (Orion Star, Thermo Fisher, USA). The operating conditions for Al by HR CS-FAAS are given in Table 1.
| Parameter | Al |
|---|---|
| Wavelength (nm) | 396.152 |
| Flow rate of N2O-C2H2 (L h—1) | 220 |
| Flow rate of C2H2-air (L h—1) | 70 |
| Burner height (mm) | 4 |
| Evaluation pixels (pm) | 3 |
| Background correction | Simultaneous |
2.2 Reagents
All solutions were prepared using ultra-pure water (specific resistance 18 MΩ cm−1) from a Milli-Q purification system (Millipore, Massachusetts, USA). Standard solutions of metals ions were prepared from the 1000 mg L−1 stock solution (Merck). Buffer solutions (Merck) of sodium acetate/acetic acid (for pH 3–5.8), sodium monohydrogen phosphate/potassium dihydrogen phosphate (for pH 6–8) and ammonium chloride/ammonia (for pH 9) were used. All glassware was cleaned using ultra-pure water, kept in nitric acid for 24 h, and washed again with ultra-pure water.
2.3 Preparation of yeast culture
C. albicans was maintained by sub-culturing on yeast extract peptone dextrose (YPD), agar slants. Inocula were obtained from seven days old agar slant cultures. The fungal mat was removed and then macerated in 10 mL of sterile YPD broth using a homogenizer. This preparation was used to inoculate 25 mL medium into a 250 mL flask, and the flasks were incubated on a shaker at 150 rpm for seven days at 35°C.
2.4 Preparation of biomass
After this incubation period, the biomass was harvested by filtration of the growth medium and was washed several times with distilled water. The fungus grown in the liquid medium was separated from the growth media by filtration through 0.45 mm filter to isolate the biomass. The fungal biomass was obtained as discrete spherical clumps; it was homogenized with a commercial blender to destroy cell aggregates. The isolated biomass was washed with doubly distilled water and dried at 80°C for about 2 h. C. albicans was stored in a refrigerator at 4°C before use.
The immobilization of C. albicans was performed according to the procedure provided in the literature 14. 500 mg of dry C. albicans powder was mixed with 5 g cellulose acetate and 4 mL doubly distilled water was added. After mixing, the paste was heated in an oven at 80°C for 2 h. The wetting and drying steps were repeated to maximize the contact between C. albicans and cellulose acetate, thereby improving the immobilization efficiency. Prepared (dry powder) CAICAB was used as a biosorbent in the column.
2.5 Preparation of separation column
A glass column of 10.0 cm length and 0.8 cm id with a 250 mL tank on top and a stopcock at the bottom of the column for separation/preconcentration of metal ions was used. The column system was prepared by placing a small portion of cleaned glass wool as a plug at one end of the column holding a certain amount (0.2–0.4 g) of adsorbent. Column system was rinsed with water, 5–10 mL of 2 mol L−1 HCl 15, 16.
2.6 Preconcentration procedure
The proposed procedure was tested with model solutions prior to the determination of trace Al in water samples. In order to prepare the model solutions 2.5 mL of 2.0 mg L−1 Al3+ solution were added to 4 mL buffer solutions (sodium acetate/acetic acid, sodium monohydrogen phosphate/potassium dihydrogen phosphate, and ammonium chloride/ammonia) (to give the desired pH between 3 and 9) in a flask. Then, the final volume was filled to 50 mL with deionized water. The column was preconditioned by passing buffer solutions of working pH. Thereafter, the model solution was passed through the column at a flow rate of 5 mL min−1. Then, the column was rinsed with 10 mL of deionized water, and the Al ions adsorbed on the CAICAB were eluted with 5 mL of 2 mol L−1 HCl. The eluent was analyzed for the determination of metal concentrations by HR-CS FAAS.
2.7 Analysis of water samples
Various water samples were collected from tap water of the research laboratory, urban wastewater treatment plants of Kırşehir city, Kızılırmak river water and dam water of Hirfanlı dam lake which is established on this river. Kırşehir is a middle huge city of Turkey and is located between the latitudes of 39°41′–39°48′N and the longitudes of 33°25′–34°43′E. Before the analysis, the samples were filtered through a cellulose membrane filter (Millipore) of pore size 0.45 µm. Buffer solution of sodium acetate/acetic acid (pH 5) and known amounts of analyte ions (10–50 µg L−1) were mixed in a volumetric flask. Then, the preconcentration procedure was applied to the obtained sample solutions.
3 Results and discussion
3.1 Scanning electron microscopy (SEM) and fourier transform IR (FTIR) spectroscopy
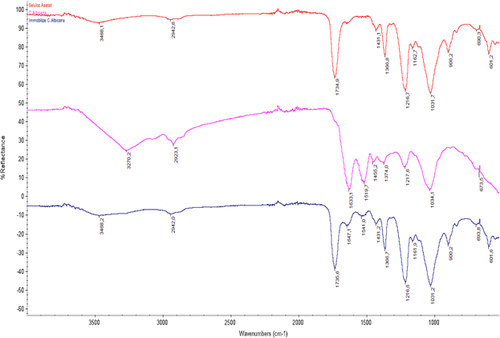
The surface morphology of C. albicans immobilized on cellulose acetate was visualized by scanning electron microscopy (Figs. 1). As can be seen in Figs. 1–3, different shapes before and after immobilization explain that immobilization on cellulose acetate occurred. The FTIR spectrum (Fig. 4) showed new absorption peaks at 1647 and 1541 cm−1 after immobilization. According to the absorption peaks, some absorption peaks of C. albicans and peaks of cellulose acetate overlap, Fig. 4, and is thought as fulfillment of immobilization on cellulose acetate.

3.2 Effect of pH on the recovery of Al
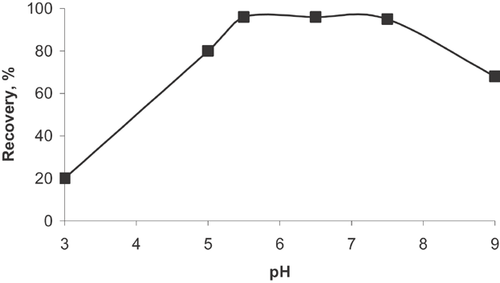
pH strongly influences the speciation and the biosorption availability of the heavy metals. Therefore, the pH of sample solutions is one of the most important experimental factors for the adsorption of toxic metal ions on biomass. The recovery of the analyte was determined by applying the preconcentration procedure by changing the pH of the model solutions in the range of 3–9. The quantitative recovery (>95%) of analyte ions was obtained at pH 5.0–7.5. The recovery of Al3+ ions was decreased when the solutions pH was >7.5 and <5.0 (Fig. 5). Hence, pH 5 was selected as an optimum pH for adsorption and recovery of the analyte ions for further experiments.
3.3 Effect of eluent type and concentration on the recovery of Al
In order to choose the most effective eluent for desorbing of Al3+ ions from the adsorbent, different concentrations and different volumes of HCl and HNO3 solutions were tested. Quantitative recovery (>95%) has been obtained by using 5 mL of 2 mol L−1 HCl (Table 2).
| Eluent | Recovery (%)a) |
|---|---|
| 1 mol L−1 HCl, 5 mL | 92 ± 1 |
| 2 mol L−1 HCl, 5 mL | 98 ± 2 |
| 1 mol L−1 HNO3, 5 mL | 90 ± 2 |
| 2 mol L−1 HNO3, 5 mL | 95 ± 2 |
- a) Results are mean ± standard deviation of three replicate analyses.
3.4 Effect of the sample flow rate on the recovery of Al
The flow rate of the sample solution also has effects on the recovery of Al ions. Therefore, the effect of flow rate was also investigated under the optimum conditions (pH 5; eluent, 5 mL of 2 mol L−1 HCl). As shown in Fig. 6, the optimum value for the flow rate of the sample solution was found as up to 5 mL min−1. Above 5 mL min−1, the recovery decreased gradually. Therefore, to decrease the duration time without decreasing recovery values, a rate of 5 mL min−1 was chosen as the optimum flow rate for subsequent experiments.
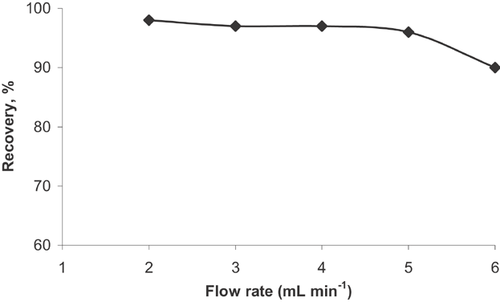
3.5 Effect of eluent flow rate on the recovery of Al
The flow rate of eluent solutions (5 mL of 2 mol L−1 HCl) on the recovery of Al3+ ions was examined in the range of 2–5 mL min−1 at optimum conditions. The recovery of analyte ions ranged between 95 and 100% at the eluent flow rate varying up to 5 mL min−1. To decrease the analysis time, the eluent flow rate was selected as 5 mL min−1.
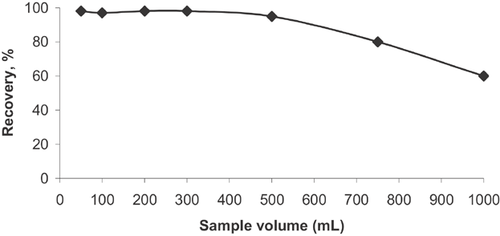
3.6 Effect of sample volume on the recovery of Al
For the preconcentration purposes, to achieve a higher preconcentration factor (PF), the eluent volume should be as small as possible, and the volume of the sample solution should as high as possible. In order to obtain the maximum applicable sample solution (or analyte concentration), model solutions including the same amount of analyte ions with different volumes were used. For this purpose, aqueous solutions containing 5 μg Al3+ were preconcentrated from sample volumes of 100, 250, 500, 750, and 1000 mL corresponding to analyte concentrations of 0.100, 0.050, 0.020, 0.010, 0.006, and 0.005 μg mL−1, respectively.
The recovery of the analyte Al3+ was quantitative (>95%) for sample volumes up to 500 mL (Fig. 7). By analyzing 5 mL of the final solutions after the preconcentration of sample solutions, a maximum PF for Al3+ was found to be as 100.
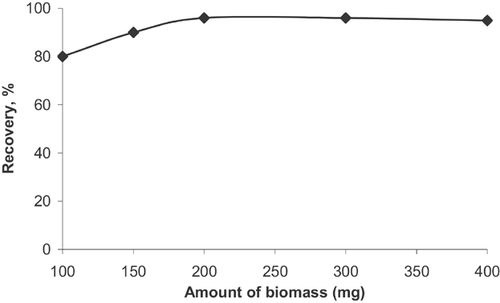
3.7 Effect of adsorbent amount
The effect of the amount of CAICAB on the recovery of the analyte was studied. For this purpose, different amounts of sorbent (100–400 mg) were tested in the adsorption column. 50 mL of a model solution including 5 µg of analyte were passed through the column at optimum experimental conditions (pH 5; flow rate 5 mL min−1; eluent, 5 mL of 2 mol L−1 HCl). The results showed that the recovery of the analytes increased up to 200 mg of CAICAB and remained stable for >200 mg of CAICAB (Fig. 8). Therefore, the minimum sorbent amount (200 mg) resulting in maximum recovery was selected for further studies.
3.8 Matrix effect on the recovery of Al
Matrix effects are important problems in adsorption, separation and recovery of heavy metals from water samples. Therefore, the effects of common coexisting ions on the recovery of Al ions were investigated. The maximum concentrations of the various coexisting metal ions as their nitrate or chloride salts which are tolerable within the 5% relative error were determined by adding them to a solution containing of analyte ions and by applying the proposed procedure. The results indicated that the added ions tested having concentrations given in Table 3 did not interfere with recovery of Al ions.
| Ion | Concentration (mg L−1) | Recovery (%)a) |
|---|---|---|
| K+ | 1000 | 96 ± 2 |
| Na+ | 2000 | 97 ± 2 |
| Ca2+ | 500 | 102 ± 2 |
| Mg2+ | 200 | 96 ± 2 |
| Zn2+ | 10 | 95 ± 2 |
| Cu2+ | 10 | 97 ± 2 |
| Co2+ | 10 | 98 ± 2 |
| Ni2+ | 10 | 98 ± 2 |
| Cd2+ | 10 | 96 ± 2 |
| Cr3+ | 10 | 103 ± 1 |
| Fe3+ | 10 | 97 ± 2 |
- a) Results are mean ± standard deviation of three replicate analyses.
3.9 Capacity of CAICAB
The adsorption capacity of the resin was calculated by the batch technique 17-19. For this process, 0.02 g CAICAB were added into a flask including 100 mL deionized water and 10 mg Al ions at the optimum pH. This mixture was stirred for 1 h with a magnetic stirrer and aluminum ions in solution were determined by HR CS-FAAS after needed dilution process (one to ten times). The calculated adsorption capacity of CAICAB for Al was 327.5 mg g−1. The capacity of the CAICAB for aluminum is higher than that of other biosorbents in the literature 11, 20-23 (Table 4). Therefore, CAICAB has an important potential for the removal, separation and preconcentration of aluminum from aqueous solution.
| Biosorbent | Sorption capacity (mg/g) | pH | Reference |
|---|---|---|---|
| Ca-loaded Laminaria japonica | 75.27 | 4.5 | 11 |
| Chryseomonas luteola TEM05 | 55.2 | 5.0 | 20 |
| Na-loaded Sargassurn fluitans | 104.14 | 4.5 | 21 |
| Typha domingensis leaf powder | 0.35 | 2.5 | 22 |
| BDH activated carbon | 6.56 | 4.0 | 23 |
| Padina pavonica | 77.30 | 4.5 | 24 |
| CAICAB | 327.5 | 5.0 | Present study |
3.10 Analytical features
Under the optimum experimental conditions, linear dynamic range, correlation coefficient, limit of detection (LOD), precision and accuracy were examined.
By using direct aspiration in HR CS-FAAS without applying the preconcentration system the linear dynamic range for Al was 0.1–20.0 mg L−1 (R2 = 0.999), respectively.
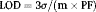
The precision of this procedure was examined by seven replicate measurements of 50 mL of sample solutions including 50 µg L−1 Al3+. The mean recovery for Al was obtained as 98.5% with the relative standard deviation, RSD, of 1.9.
3.11 Reusability of the sorbent
The stability and reusability of the sorbent were evaluated by determining the recovery of the analyte by applying adsorption–elution cycles. One adsorption–elution cycle was considered as: passage of 50 mL of the model solution, 5 mL eluent solution and 50 mL ultra-pure water through the column loaded with 200 mg of sorbent, respectively. The capacity of the adsorbent did not significantly change up to 50 cycles.
3.12 Analytical applications
In order to show the applicability of the method, it was applied for the determination of Al in water samples at optimized conditions. The accuracy of the method was checked by determining the percent relative error of spiked real samples. The results obtained are given in Table 5. A good agreement was obtained between added and found value of the analytes.
| Sample | Added (μg L−1) | Founda) (μg L−1) | Relative error (%) |
|---|---|---|---|
| Tap water | 0 | 22.8 ± 1.6 | |
| 10.0 | 33.6 ± 2.4 | 2.4 | |
| River water | 0 | 78.5 ± 5.2 | |
| 50.0 | 125 ± 8.4 | −2.7 | |
| Wastewater | 0 | 724 ± 25 | |
| 200 | 953 ± 32 | 3.1 | |
| Dam water | 0 | 42.9 ± 3.8 | |
| 20.0 | 60.4 ± 7.6 | −3.9 |
- a) Mean and standard deviation from three determinations.
4 Conclusions
In this study, not only CAICAB was obtained as a new biosorbent but also a new analytical method was developed for the separation and preconcentration of aluminum ions in various water samples. The developed method has good reproducibility, accuracy, high PF, and low detection limit. The method was successfully applied to the determination of aluminum in different water samples.
Acknowledgments
The authors would like to express appreciation for the support of the sponsors (The Scientific and Technological Research Council of Turkey [TUBITAK] Grant no: 110T111 and Ahi Evran University Grant no: PYO-FEN.4003.13.4007).
The authors have declared no conflict of interest.




