Predictive value of serum iron on heart failure in patients with acute ST-segment elevation myocardial infarction
Wen Chen and Guoli Lin contributed equally to this work and should be considered cofirst authors.
Abstract
Background
In clinical practice, heart failure often occurs after acute myocardial infarction, and a new biomarker for its early prediction is urgently needed. The aim of this study was to investigate the relationship between serum iron and heart failure after acute ST-segment elevation myocardial infarction (STEMI).
Methods
A total of 41 patients with heart failure after STEMI and 31 controls were included in the study. The demographic variables and baseline clinical characteristics of both groups were analyzed.
Results
There were no significant differences between patients with heart failure and controls in terms of demographic characteristics. There were significant differences in terms of serum iron, N terminal pro-B-type natriuretic peptide levels, left atrial diameter, and left ventricular ejection fraction. Binary logistic regression analyses demonstrated that serum iron (odds ratio [OR]: 0.804, 95% confidence interval [CI]: 0.699–0.924) and Tn-I (OR: 1.072, 95% CI: 1.011–1.137) were independent predictors for heart failure (p < .05, respectively). Receiver operating characteristic analysis showed that the area under the curve for serum iron was 0.808 (95% CI: 0.707–0.908, p < .01). The best cutoff value of serum iron was 11.87 μmol/L (sensitivity: 87.1%; specificity: 68.3%).
Conclusions
Patients with heart failure after STEMI have lower serum iron levels than patients without heart failure after STEMI. Serum iron levels are a risk factor for heart failure after STEMI.
1 INTRODUCTION
Heart failure (HF) is a serious and terminal stage of cardiovascular disease, with high morbidity and mortality. With the aging of the population, the prevalence of HF is also increasing, and is increasingly becoming an important global public health problem.1 Myocardial infarction (MI) is an important risk for HF. There is a high incidence of postinfarction HF in China but insufficient information on the epidemiology. Currently, the prognosis of patients with postinfarction HF has improved, but all-cause mortality, cardiovascular event rates, and rehospitalization rates remain high, which is related to the severity of the disease and whether there is timely diagnosis and treatment of these patients.2 Iron catalyzes redox reactions that maintain cell balance or drive toxic damage. It not only plays a central role in cardiac energetics, but can also cause the death of cardiac muscle cells through uncontrolled oxidative stress.3 One study directly analyzed myocardial tissue samples from patients with and without HF and found that myocardial iron levels were lower in patients with HF. This was associated with reduced mitochondrial enzyme activity in HF patients, suggesting that iron plays a key role in myocardial function.4 Patients with HF combined with iron deficiency have deteriorated muscle function due to reduced mitochondrial iron stores in myocytes, resulting in decreased cardiac function.5 The incidence of HF after acute MI is increasing. Clinical observation found that the occurrence of HF was more than 24 h after acute MI. Iron catalyzes redox reactions that maintain cell balance or drive toxic damage. It not only plays a central role in cardiac energetics, but can also cause the death of cardiac muscle cells through uncontrolled oxidative stress.3 One study directly analyzed myocardial tissue samples from patients with and without HF and found that myocardial iron levels were lower in patients with HF. This was associated with reduced mitochondrial enzyme activity in HF patients, suggesting that iron plays a key role in myocardial function.4 Patients with HF combined with iron deficiency have deteriorated muscle function due to reduced mitochondrial iron stores in myocytes, resulting in decreased cardiac function.5 It had been shown that reduced serum iron levels in patients with acute HF are associated with poor prognosis, including 12-month mortality.6 Ye Gang et al. showed that A lower admission serum iron level is an independent predictor of acute HF in STEMI patients during hospitalization.7 The study also shown that low serum iron is an independent predictor of poor prognosis for acute decompensated HF regardless of hemoglobin or ferritin levels.8 Determining whether serum iron levels can predict HF after STEMI could improve early recognition and management. The incidence of HF after acute MI is increasing. Clinical observation found that the occurrence of HF was more than 24 h after acute MI. Therefore, sensitive indicators are needed to assess risk factors for HF before onset. The aim of our study was to determine the association of serum iron with HF in patients with acute ST-segment elevation MI.
2 METHODS
2.1 Study design and populations
Patients with acute ST-segment elevation myocardial infarction (STEMI) hospitalized in the Department of Cardiovascular Medicine from December 2021 to May 2022 were selected. STEMI patients with new onset of HF during hospitalization were the HF group. STEMI patients with no HF during hospitalization were control group. Exclusion criteria included: Pre-existing HF; a history of previous infarction; recent acute infection or chronic inflammatory disease; malignant tumor; valvular heart disease; anemia; restrictive and hypertrophic cardiomyopathy; currently taking iron medication and Hepatic disease. The diagnosis of acute ST-segment elevation MI was in compliance with the guidelines for the diagnosis and treatment of acute ST-segment elevation MI published by the Chinese Medical Association in 2019 (Supporting Information: 1).9 The diagnosis of HF after MI mainly depended on the history, symptoms, signs and auxiliary examination, and was in line with the criteria of the 2020 Expert Consensus on the Prevention and Treatment of Heart Failure After Myocardial Infarction (Supporting Information: 2).1
2.2 Data collection
All data were obtained from the hospital's digital information system. Baseline demographic and clinical characteristics of all patients were recorded, including information on age, gender, hypertension, and diabetes. The normal range of each indicator. Serum iron (12–30 μmol/L), N terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels (300–1800 Pg/mL), total cholesterol (TC) (1.6–.17 mmol/L), high-density lipoprotein cholesterol (HDL-C) (1.16–1.55 mmol/L), low-density lipoprotein cholesterol (LDL-C) (2.6–3.4 mmol/L), uric acid (155–357 μmol/L), alanine aminotransferase (ALT) (7–40 IU/L), aspartate aminotransferase (AST) (13–40 IU/L), hemoglobin (130–175 g/L), d-dimer (0–0.5 μg/mL), c-reactive protein (CRP) (0–10 mg/L), troponin I (Tn-I) (0–0.03 ng/mL).
2.3 Clinical measurements
On admission, blood samples were placed through an anterior axillary venipuncture into an ethylenediaminetetraacetic acid-treated test tube or a plain test tube. Complete blood counts were measured using an automated hematological analyzer (CAL8000; Mindray Corporation). The levels of NT-pro-BNP and Tn-I were measured using an automated fluorescent immunoassay (Getein1600; Geteinbiotech). Blood biochemical examination and serum iron were measured using an automated biochemical analyzer (CM-800; Geteinbiotech).
2.4 Echocardiography
Echocardiography was performed in all patients using a Vivid 7 echocardiography device (General Electric). All patients underwent two-dimensional, M-mode, and Doppler echocardiography.
2.5 Statistical analysis
The Kolmogorov–Smirnov test was used to demonstrate the normality of the included variables, and p > .05 was defined as normally distributed data. Continuous variables, expressed as mean ± standard deviation, were compared between two normally distributed continuous variables using the independent samples t test, while the Mann–Whitney U test was used to compare differences between non-normally distributed continuous variables. Categorical variables, expressed as frequencies and percentages, were compared using χ2 tests. Binary logistic regression analysis was used to identify potential independent associations between postinfarction HF and clinical parameters. Receiver operating characteristic (ROC) curves were analyzed to identify the best cutoff values for the prediction of postinfarction HF. p < .05 represents a statistically significant difference (two-tailed test). All statistical analyses were conducted using the Statistical Package for Social Sciences software (SPSS 19.0 for Windows; IBM).
3 RESULTS
3.1 Patient characteristics
A total of 41 patients with HF (80.5% males; mean age: 64.49 ± 12.09 years) and 31 controls (70.9% males; mean age: 63.84 ± 9.83 years) were included in the study. The demographic and clinical characteristics of the patients are summarized in Table 1. There were no significant differences between patients with HF and controls in terms of demographic characteristics.
| Variables | Heart failure (n = 41) | Control (n = 31) | p |
|---|---|---|---|
| Age (years) | 64.49 ± 12.09 | 63.84 ± 9.83 | .808 |
| BMI (kg/m2) | 24.6 ± 3.86 | 24.4 ± 3.90 | .542 |
| Male gender (%) | 33 (80.5%) | 22 (70.9%) | .407 |
| Hypertension (%) | 26 (63.4%) | 17 (54.8%) | .637 |
| Diabetes mellitus (%) | 18 (43.9%) | 7 (22.5%) | .069 |
| Culprit lesions | |||
| LM (%) | 1 (2.4%) | 1 (3.2%) | .679 |
| LAD (%) | 16 (39%) | 8 (25.8%) | .315 |
| LCX (%) | 16 (39%) | 13 (41.9) | .813 |
| RCA (%) | 8 (19.5%) | 9 (29%) | .407 |
| Smoker (%) | 20 (48.8%) | 15 (48.4%) | .581 |
| Onset-to-ED (min) | 425.36 ± 169.79 | 410.96 ± 171.80 | .724 |
| D-to-W (min) (min) | 68.51 ± 10.36 | 66.35 ± 9.76 | .373 |
- Abbreviations: BMI, body mass index; D-to-W, door-to-wire; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main coronary artery; Onset-to-ED, onset-to-emergency department; RCA, right coronary artery.
3.2 Comparison of clinical and echocardiographic parameters between the two groups
There were significant differences in serum Iron (11.35 ± 5.58 vs. 16.01 ± 4.26), NT-pro-BNP levels (4883.17 ± 7695.15 vs. 493.32 ± 360.39), left anterior descending artery (39.98 ± 7.24 vs. 35.97 ± 5.04), and left ventricular ejection fraction (52.03 ± 10.74 vs. 58.43 ± 7.35) between the HF and control group. There were no significant differences in terms of TC, HDL-C, LDL-C, uric acid, ALT, AST, hemoglobin, d-dimer, CRP, peak Tn-I (Table 2).
| Variables | Heart failure (n = 41) | Control (n = 31) | p |
|---|---|---|---|
| TC (mmol/L) | 4.66 ± 1.53 | 4.25 ± 1.08 | .184 |
| HDL-C (mmol/L) | 1.01 ± 0.17 | 1.11 ± 0.31 | .090 |
| LDL-C (mmol/L) | 3.17 ± 1.35 | 2.81 ± 0.92 | .206 |
| Uric acid (μmol/L) | 417.35 ± 134.54 | 438.98 ± 103.88 | .406 |
| ALT (IU/L) | 37.67 ± 26.57 | 30.95 ± 23.51 | .268 |
| AST (IU/L) | 52.63 ± 49.31 | 42.23 ± 35.71 | .303 |
| Serum iron (μmol/L) | 11.35 ± 5.58 | 16.01 ± 4.26 | .001 |
| Hemoglobin (g/L) | 141.97 ± 14.27 | 137.42 ± 15.66 | .203 |
| d-dimer (μg/mL) | 1.08 ± 1.46 | 0.84 ± 1.12 | .476 |
| CRP (mg/L) | 14.86 ± 16.91 | 12.08 ± 27.38 | .616 |
| Peak NT-pro-BNP (pg/ml) | 4883.17 ± 7695.15 | 493.32 ± 360.39 | .002 |
| Peak Tn-I (ng/ml) | 14.57 ± 10.98 | 10.31 ± 9.41 | .088 |
| LAD (mm) | 39.98 ± 7.24 | 35.97 ± 5.04 | .044 |
| LVEF (%) | 52.03 ± 10.74 | 58.43 ± 7.35 | .007 |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LAD, left atrial diameter; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; Nt-pro-BNP, N terminal pro B type natriuretic peptide; TC, total cholesterol; Tn-I, troponin I.
3.3 Predictors of HF
Binary logistic regression analyses demonstrated that serum iron and Tn-I were independent predictors for HF (p < .05, respectively) (Table 3).
| Variables | β | Wals | p | OR | 95% CI |
|---|---|---|---|---|---|
| Serum iron | −.219 | 9.360 | .002 | 0.804 | 0.699–0.924 |
| Tn-I | .069 | 5.335 | .021 | 1.072 | 1.011–1.137 |
| LAD | −.063 | 1.578 | .209 | 1.065 | 0.965–1.176 |
| Age | .001 | 0.001 | .987 | 1.001 | 0.949–1.055 |
- Abbreviations: CI, confidence interval; LAD, left atrial diameter; OR, odds ratio: Tn-I, troponin I.
3.4 ROC curve
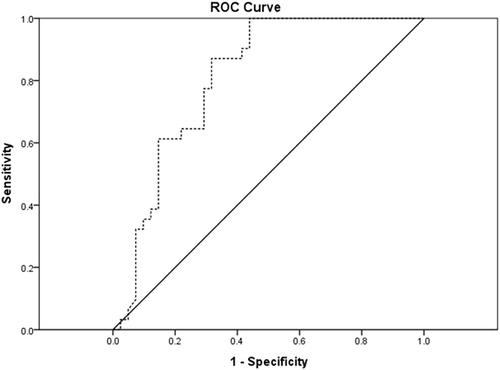
For the prediction of HF, ROC analysis showed that the area under the curve for serum iron was 0.808 (95% confidence interval [CI]: 0.707–0.908, p < .01). The best cutoff value of serum iron was 11.87 μmol/L (sensitivity: 87.1%; specificity: 68.3%) (Figure 1).
4 DISCUSSION
This paper is a retrospective case–control study looking at the predictive value of serum iron levels on HF after STEMI involving 41 patients at a single medical center in China. Our study showed that serum iron levels were lower in patients with HF than in controls, but the left atrium was larger than in controls. Serum iron and Tn-I were independently associated with HF. In addition, ROC analysis revealed that the best cutoff value of serum iron to predict HF occurrence was 11.87 μmol/L with a sensitivity of 87.1% and a specificity of 68.3%.
There was relatively little information on the epidemiology of postinfarction HF in China. The BRIGHT study found that the incidence of HF on admission in patients with acute infarction who underwent emergency PCI was 14.3%, of whom 88% were STEMI patients.10 Data from the China PEACE study showed that the incidence of in-hospital early onset HF in STEMI patients was decreasing from 2001 to 2011, but was still 12.7%.2 Myocardial cell loss was an important cause of cardiac remodeling and the development of HF after infarction. Cardiomyocytes in the ischemic zone activated apoptotic signaling pathways in response to oxidative stress, inflammatory response, and other injury factors, which in turn mediated cardiomyocyte apoptosis.11 Iron was involved in the synthesis and function of a variety of enzymes and proteins involved in oxygen transport and storage, electron transfer, and oxidation–reduction.12 Iron deficiency leaded to a reduction in mitochondrial oxidative capacity.13 Decreased cardiac function may be caused by a systemic iron deficiency that compromises the cardiac energy metabolism.14 Studies had shown that patients with HF had reduced myocardial iron levels, leading to decreased myocardial function.15 Iron had also been shown to be involved in the pathogenesis of atherosclerotic coronary artery disease.16 Free iron promoted the oxidation of low-density lipoprotein. Uptake of low-density lipoprotein by low-density lipoprotein receptors on macrophages leaded to the recruitment of foam cells, whose infiltration and necrotic core expansion were key to coronary atherosclerosis.17 Serum iron concentration was a measure of circulating iron bound to transferrin and serves as a proxy for iron status. Serum iron may not be as easily stored as serum ferritin, but it is a measure of iron supply to bone marrow and other tissues, and is one of the primary biochemical indicators of iron status. Moreover, serum iron had shown a strong negative correlation between iron concentrations and cardiovascular disease.18 Several studies had shown that serum iron levels may be a good marker for short-term risk of cardiovascular disease in older adults.19-21 The PREDIMED trial shown that, among Spanish adults aged 55–80 years at high cardiovascular disease risk, low iron concentrations in serum were associated with increased short-term risk of cardiovascular disease, and these associations were stronger in women.22 Although the relationship between serum iron deficiency and myocardial iron deficiency is not fully understood, systemic iron deficiency was observed in two of the last three patients with HF.14 Moreover, iron deficiency in MI predicts a poor prognosis.23 In patients with a first anterior ST-segment elevation MI, iron deficiency was associated with larger infarcts, more extensive microvascular obstruction, and higher frequency of adverse left ventricular remodeling. The possible mechanisms were iron deficiency decreased endothelial nitric oxide synthase/soluble guanylate cyclase/protein kinase G pathway activity associated with oxidative/nitrosative stress and increased infarct size after transient coronary occlusion.24
There are some limitations in this article. First, This study is a retrospective study, and the population is small. Second, the interventional approach to acute MI may have an impact on the prognosis of HF. Third, whether basal serum iron levels in patients are associated with HF needs further confirmation. Finally, further large-scale prospective studies are needed to clarify the role of serum iron in predicting HF after acute MI.
5 CONCLUSIONS
Patients with HF after STEMI have lower serum iron levels than patients without HF after STEMI. Serum iron levels are a risk factor for HF after STEMI. Though the study is small, it could lead to further studies on the relationship between iron levels, cardiovascular events, and decisions on management.
ACKNOWLEDGMENTS
The authors are grateful to all individuals who participated in this research.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request to the corresponding author due to privacy/ethical restrictions.




