Efficient Allylation of Dihalides: A Versatile Approach to C/N/O-Functionalized Derivatives
Mengdi Pang
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorWentao Hao
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorXiulin Li
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorChunyan Zhang
School of Environmental and Engineering, Taiyuan University of Technology, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorAli Morsali
Department of Chemistry, Tarbiat Modares University, Tehran, 14115-4838 Iran
Search for more papers by this authorAli Ramazani
The Organic Chemistry Research Laboratory (OCRL), Department of Chemistry, Faculty of Science, University of Zanjan, Zanjan, 45371-38791 Iran
Search for more papers by this authorCorresponding Author
Guoying Zhang
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
E-mail: [email protected].Search for more papers by this authorMengdi Pang
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorWentao Hao
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorXiulin Li
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorChunyan Zhang
School of Environmental and Engineering, Taiyuan University of Technology, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorAli Morsali
Department of Chemistry, Tarbiat Modares University, Tehran, 14115-4838 Iran
Search for more papers by this authorAli Ramazani
The Organic Chemistry Research Laboratory (OCRL), Department of Chemistry, Faculty of Science, University of Zanjan, Zanjan, 45371-38791 Iran
Search for more papers by this authorCorresponding Author
Guoying Zhang
Institute of Coal Chemistry, State Key Laboratory of Coal Conversion, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
E-mail: [email protected].Search for more papers by this authorComprehensive Summary
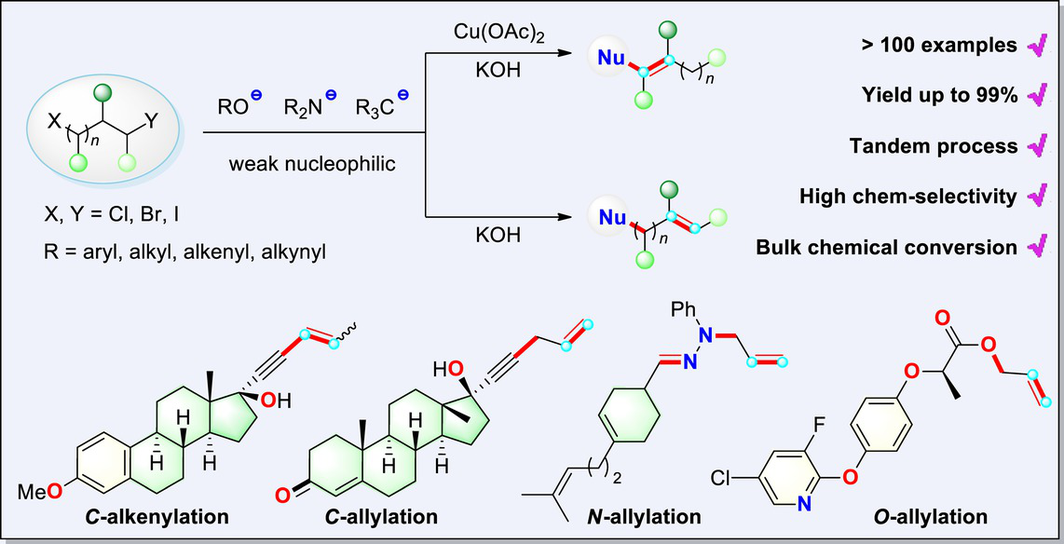
This study presents an efficient and innovative allylation strategy utilizing C/N/O nucleophilic reagents with attenuated reactivity, enabling the construction of versatile allyl compounds. The approach focuses on the sequential allylation of dihalides in large-scale chemical manufacturing, effectively addressing the challenge of achieving selectivity in cascade reactions. The methodology is centered on the Cu-catalyzed C-olefination of alkynes with dihalides, significantly expediting the synthesis of a diverse array of finely conjugated enyne derivatives. Furthermore, a base-facilitated sequential condensation process has been developed to achieve the N-allylation of hydrazines, yielding a wide range of trisubstituted alkenyl hydrazones. Additionally, the protocol enables the synthesis of high-value ester compounds through O-allylation or esterification with dihalides. This transformation also facilitates the one-step synthesis of a variety of essential pharmaceuticals, demonstrating its broad synthetic utility and potential.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500212-sup-0001-supinfo.pdfPDF document, 12.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Shi, T. M.; Chen, X. F.; Ti, H. H. Ferroptosis-Based Therapeutic Strategies toward Precision Medicine for Cancer. J. Med. Chem. 2024, 67, 2238–2263; (b) Piñeiro-Suárez, M.; Alvarez-Constantin, A. M.; Fañanás-Mastral, M. Copper-Catalyzed Enantioselective Borylative Allyl-Allyl Coupling of Allenes and Allylic gem-Dichlorides. Acs Catal. 2023, 13, 5578–5583; (c) Sun, J.; Ye, H.; Sun, F.; Pan, Y. Y.; Zhu, X. W.; Wu, X. X. Palladium-Catalyzed Allylation of P(O)H Compounds: Access to 2-Fluoroallylic Phosphorus Compounds. Org. Lett. 2023, 25, 5220–5225; (d) Zhao, D.; Xu, B.; Zhu, C. Migratory allylic arylation of 1,n-enols enabled by nickel catalysis. Nat. Commun. 2023, 14, 3308; (e) Zhu, J.; Wang, J. X.; Wang, X.; Gao, M. J.; Guo, B. B.; Gao, M. M.; Liu, J. R.; Yu, Y. Q.; Wang, L.; Kong, W. K. X.; An, Y. P.; Liu, Z. R.; Sun, X. P.; Huang, Z.; Zhou, H.; Zhang, N.; Zheng, R. M.; Xie, Z. W. Prediction of drug efficacy from transcriptional profiles with deep learning. Nat. Biotechnol. 2021, 39, 1444–1452; (f) Teo, W. J.; Yang, X. X.; Poon, Y. Y.; Ge, S. Z. Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes. Nat. Commun. 2020, 11, 5193; (g) Chianese, G.; Sirignano, C.; Shokoohinia, Y.; Mohammadi, Z.; Bazvandi, L.; Jafari, F.; Jalilian, F.; Moriello, A. S.; De Petrocellis, L.; Taglialatela-Scafati, O.; Rigano, D. TRPA1 Modulating C14 Polyacetylenes from the Iranian Endemic Plant. Molecules 2018, 23, 1750; (h) Mata, S.; López, L. A.; Vicente, R. Synthesis of Bifunctional Allylic Compounds by Using Cyclopropenes as Functionalized Allyl Equivalents. Angew. Chem. Int. Ed. 2018, 57, 11422–11426.
- 2(a) Wang, X.; Xu, Y.; Wang, J. B. Efficient Dienyl End-Capping of Ruthenium Catalyzed Ring Opening Metathesis Polymerization with Allyl Compounds through Base-Promoted Metallacyclobutane Decomposition. Angew. Chem. Int. Ed. 2024, 63, e202409534; (b) Jung, Y. S.; Yoo, S. Y.; Jin, Y. H.; You, J. H. Y.; Han, S. C.; Yu, J. W.; Park, Y.; Cho, S. H. Iridium-Catalyzed Chemo-, Diastereo-, and Enantioselective Allyl- Allyl Coupling: Accessing All Four Stereoisomers of (E)-1-Boryl-Substituted 1,5-Dienes by Chirality Pairing. Angew. Chem. Int. Ed. 2023, 62, e202218794; (c) Wang, T. C.; Wang, P. S.; Chen, D. F.; Gong, L. Z. Access to chiral homoallylic vicinal diols from carbonyl allylation of aldehydes with allyl ethers palladium-catalyzed allylic C-H borylation. Sci. China Chem. 2022, 65, 298–303; (d) Liu, X. J.; Zhou, S. Y.; Xiao, Y. T.; Sun, Q.; Lu, X.; Li, Y.; Li, J. H. Photocatalytic Decarboxylative [3+2] and [4+2] Annulation of Enynals and γ,σ-Unsaturated-(Acyloxy)phthalimides by NaI/PPh3 Catalysis. Org. Lett. 2021, 23, 7839–7844; (e) Chen, Y. W.; Liu, Y.; Lu, H. Y.; Lin, G. Q.; He, Z. T. Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization. Nat. Commun. 2021, 12, 5626; (f) Ye, F.; Ge, Y.; Spannenberg, A.; Neumann, H.; Beller, M. The role of allyl ammonium salts in palladium-catalyzed cascade reactions towards the synthesis of spiro-fused heterocycles. Nat. Commun. 2020, 11, 5383; (g) Wu, Z. J.; Li, S. R.; Xu, H. C. Synthesis of N-Heterocycles by Dehydrogenative Annulation of N-Allyl Amides with 1,3-Dicarbonyl Compounds. Angew. Chem. Int. Ed. 2018, 57, 14070–14074; (h) Höfler, D.; van Gemmeren, M.; Wedemann, P.; Kaupmees, K.; Leito, I.; Leutzsch, M.; Lingnau, J. B.; List, B. 1,1,3,3-Tetratriflylpropene (TTP): A Strong, Allylic C-H Acid for Bronsted and Lewis Acid Catalysis, Angew. Chem. Int. Ed. 2017, 56, 1411–1415.
- 3(a) Fischbach, D. M.; Krstic, K. A.; Sita, L. R. Versatile Production of Multivariate, Hyperdimensional End Group and Main Chain Functionalized Polyolefins. Angew. Chem. Int. Ed. 2023, 62, e202304725; (b) Deng, G. G.; Duan, S. Z.; Wang, J.; Chen, Z.; Liu, T. Q.; Chen, W.; Zhang, H. B.; Yang, X. D.; Walsh, P. J. Transition-metal-free allylation of 2-azaallyls with allyl ethers through polar and radical mechanisms. Nat. Commun. 2021, 12, 3860; (c) Liu, X. F.; Liu, B. X.; Liu, Q. Migratory Hydrogenation of Terminal Alkynes by Base/Cobalt Relay Catalysis. Angew. Chem. Int. Ed. 2020, 59, 6750–6755.
- 4(a) Vázquez-Galiñanes, N.; Sciortino, G.; Piñeiro-Suárez, M.; Tóth, B. L.; Maseras, F.; Fañanás-Mastral, M. Switching Selectivity in Borylative Allyl-Allyl Cross-Coupling through Synergistic Catalysis. J. Am. Chem. Soc. 2024, 146, 21977–21988; (b) Bartolo, N. D.; Read, J. A.; Valentín, E. M.; Woerpel, K. A. Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C=N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin- Anh and Chelation-Control Models. Chem. Rev. 2020, 120, 1513–1619; (c) Scharnagel, D.; Goller, J.; Deibl, N.; Milius, W.; Breuning, M. The Enantioselective Total Synthesis of Bisquinolizidine Alkaloids: A Modular "Inside-Out" Approach. Angew. Chem. Int. Ed. 2018, 57, 2432–2435; (d) Oslovsky, V. E.; Solyev, P. N.; Polyakov, K. M.; Alexeev, C. S.; Mikhailov, S. N. Chemoenzymatic synthesis of cytokinins from nucleosides: ribose as a blocking group. Org. Biomol. Chem. 2018, 16, 2156–2163.
- 5(a) Zeng, Y. X.; Gao, H.; Jiang, Z. T.; Zhu, Y. L.; Chen, J. Q.; Zhang, H.; Lu, G.; Xia, Y. Observation of unusual outer-sphere mechanism using simple alkenes as nucleophiles in allylation chemistry. Nat. Commun. 2024, 15, 4317; (b) Yao, J.; Shao, L. L.; Huo, X. H.; Wang, X. M. Synergistic silver-mediated and palladium-catalyzed nondirected olefination of aryl C-H bond: quick access to multi-substituted aryl olefins. Sci. China Chem. 2024, 67, 882–889; (c) Zi, Y.; Lange, M.; Schultz, C.; Vilotijevic, I. Latent Nucleophiles in Lewis Base Catalyzed Enantioselective N-Allylations of N-Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 10727–10731.
- 6(a) Aragón, J.; Sun, S. Y.; Pascual, D.; Jaworski, S.; Lloret-Fillol, J. Photoredox Activation of Inert Alkyl Chlorides for the Reductive Cross- Coupling with Aromatic Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202114365; (b) Acosta-Guzmán, P.; Rodríguez-López, A.; Gamba- Sánchez, D. Pummerer Synthesis of Chromanes Reveals a Competition between Cyclization and Reductive Chlorination. Org. Lett. 2019, 21, 6903–6908.
- 7(a) Wang, C. T.; Wu, R.; Chen, K.; Zhu, S. F. Enantioselective Synthesis of Biscyclopropanes Using Alkynes as Dicarbene Equivalents. Angew. Chem. Int. Ed. 2023, 62, e202305864; (b) Zhou, N. N.; Xia, Z. Q.; Kuang, K. M.; Xu, Q. K.; Zhao, F. L.; Wang, L.; Zhang, M. Visible- Light-Induced Difluoroalkylation of 1-(Allyloxy)-2-(1-arylvinyl)benzenes and 1-(1-Arylvinyl)-2-(vinyloxy)benzenes: Synthesis of Bis-Difluoroalkylated Benzoxepines and 2H-Chromenes. Org. Lett. 2022, 24, 5791–5796; (c) Chen, H.; Yan, Y. Y.; Zhang, N. N.; Mo, Z. Y.; Xu, Y. L.; Chen, Y. Y. Visible-Light-Induced Cyclization/Aromatization of 2-Vinyloxy Arylalkynes: Synthesis of Thio-Substituted Dibenzofuran Derivatives. Org. Lett. 2021, 23, 376–381.
- 8(a) Mills, L. R.; Simmons, E. M.; Lee, H. J.; Nester, E.; Kim, J.; Wisniewski, S. R.; Pecoraro, M. V.; Chirik, P. J. (Phenoxyimine)nickel- Catalyzed C(sp2)-C(sp3) Suzuki-Miyaura Cross-Coupling: Evidence for a Recovering Radical Chain Mechanism. J. Am. Chem. Soc. 2024, 146, 10124–10141; (b) Canfield, A. M.; Rodina, D.; Paradine, S. M. Dienes as Versatile Substrates for Transition Metal-Catalyzed Reactions. Angew. Chem. Int. Ed. 2024, 63, e202401550; (c) Wang, K.; Kong, W. Q. Synthesis of Fluorinated Compounds by Nickel-Catalyzed Defluorinative Cross-Coupling Reactions. ACS Catal. 2023, 13, 12238–12268; (d) Gao, J.; He, X. C.; Liu, Y. L.; Li, K. R.; Guan, J. P.; Chen, H. B.; Xiang, H. Y.; Chen, K.; Yang, H. Visible-Light-Induced Nickel-Catalyzed Cross-Coupling of Aryl Bromides with Nitriles. Org. Lett. 2023, 25, 8824–8828; (e) Liu, L. X.; Dong, J. Y.; Fu, Z. Q.; Su, L. B.; Wu, S. F.; Shang, Q.; Yin, S. F.; Zhou, Y. B. Specific cross-dimerization of terminal alkynes via Pd/TMEDA catalysis. Sci. China Chem. 2022, 65, 2487–2493.
- 9(a) Xu, K.; Zhang, Y. Y.; Wang, W. W.; Peng, M.; Liu, J. C.; Ma, C.; Zhang, Y. W.; Jia, C. J.; Ma, D.; Yan, C. H. Single-Atom Barium Promoter Enormously Enhanced Non-Noble Metal Catalyst for Ammonia Decomposition. Angew. Chem. Int. Ed. 2025, 64, e202416195; (b) Chen, H. D.; Yang, W. H.; Zhang, J. Y.; Lu, B.; Wang, X. M. Divergent Geminal Alkynylation-Allylation and Acylation-Allylation of Carbenes: Evolution and Roles of Two Transition-Metal Catalysts. J. Am. Chem. Soc. 2024, 146, 4727–4740; (c) Ren, Y. Y.; Yang, Y. S.; Wei, M. Recent Advances on Heterogeneous Non-noble Metal Catalysts toward Selective Hydrogenation Reactions. ACS Catal. 2023, 13, 8902–8924; (d) Arundhathi, K. V.; Vaishnavi, P.; Aneeja, T.; Anilkumar, G. Copper- catalyzed Sonogashira reactions: advances and perspectives since 2014. Rsc Adv. 2023, 13, 4823–4834; (e) Magre, M.; Szewczyk, M.; Rueping, M. s-Block Metal Catalysts for the Hydroboration of Unsaturated Bonds. Chem. Rev. 2022, 122, 8261–8312; (f) Mo, X. L.; Huang, H.; Zhang, G. Z. Tetrasubstituted Carbon Stereocenters via Copper-Catalyzed Asymmetric Sonogashira Coupling Reactions with Cyclic gem-Dihaloketones and Tertiary α-Carbonyl Bromides. ACS Catal. 2022, 12, 9944–9952; (g) Mo, X. L.; Chen, B.; Zhang, G. Z. Copper-Catalyzed Enantioselective Sonogashira Type Coupling of Alkynes with α-Bromoamides. Angew. Chem. Int. Ed. 2020, 59, 13998–14002; (h) Thomas, A. M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698.
- 10 Hao, W. T.; Gao, S.; Cui, H. Y.; Ding, D.; Jiang, S. H.; Zhang, C. Y.; Ji, Y. Q.; Zhang, G. Y. Construction of Trisubstituted Hydrazones via Base- Mediated Cascade Condensation N-Alkylation. J. Org. Chem. 2024, 89, 2605–2621.
- 11(a) Wang, F. C.; Ding, D.; Zhang, C. Y.; Zhang, G. Y. Formation of ylidenehydrazines enabled by manganese-catalyzed acceptorless dehydrogenative coupling. Org. Chem. Front. 2024, 11, 1420–1429; (b) Zhang, C. Y.; Liang, Z. Y.; Jia, X. F.; Wang, M. R.; Zhang, G. Y.; Hu, M. L. A practical base mediated synthesis of 1,2,4-triazoles enabled by a deamination annulation strategy. Chem. Commun. 2020, 56, 14215–14218.
- 12(a) Liang, Q. Q.; Gao, S.; Hao, W. T.; Zhang, G. Y. Palladium-Catalyzed Multicomponent Carbonylation of Halides to Acylhydrazones. J. Org. Chem. 2024, 89, 8537–8545; (b) Liang, Q. Q.; Cai, Y.; Jiang, W. J.; Pang, M. D.; Fan, L. M.; Zhang, G. Y. Palladium-catalyzed allylation and carbonylation: access to allylhydrazones and allyl acylhydrazones. Chem. Commun. 2024, 60, 1638–1641; (c) Jiang, W. J.; Pang, M. D.; Cai, Y.; Zhang, G. H.; Liu, Y. R.; Zhang, G. Y. Four-component regio- divergent carbonylative condensations for the sustainable syntheses of acylhydrazones. Org. Chem. Front. 2024, 11, 3833–3841; (d) Gao, S.; Hao, W. T.; Ji, Y. Q.; Li, X. L.; Zhang, C. Y.; Zhang, G. Y. Co-Catalyzed Dehydrogenation Claisen Condensation of Secondary Alcohols with Esters. Chin. J. Chem. 2024, 42, 2818–2824.
- 13 Ji, J. L.; Huo, Y. H.; Dai, Z. W.; Chen, Z. N.; Tu, T. Manganese-Catalyzed Mono-N-Methylation of Aliphatic Primary Amines without the Requirement of External High-Hydrogen Pressure. Angew. Chem. Int. Ed. 2024, 63, e202318763.
- 14(a) Mukherjee, S.; Aoki, Y.; Kawamura, S.; Sodeoka, M. Ligand-Controlled Copper-Catalyzed Halo-Halodifluoromethylation of Alkenes and Alkynes Using Fluorinated Carboxylic Anhydrides. Angew. Chem. Int. Ed. 2024, 63, e202407150; (b) Mo, X. L.; Huang, H.; Zhang, G. Z. Tetrasubstituted Carbon Stereocenters via Copper-Catalyzed Asymmetric Sonogashira Coupling Reactions with Cyclic gem-Dihaloketones and Tertiary α-Carbonyl Bromides. ACS Catal. 2022, 12, 9944–9952.
- 15(a) Wang, Q. Z.; Wang, M. N.; Wu, Q. H.; Ma, M. T.; Zhao, B. L. Synthesis of β-Polychlorinated Alkynes Enabled by Copper-Catalyzed Multicomponent Reaction. Org. Lett. 2022, 24, 4772–4777; (b) Fan, S. L.; Zheng, C. G.; Zheng, K. T.; Li, J. L.; Liu, Y. M.; Yan, F. P.; Xiao, H.; Feng, Y. S.; Zhu, Y. Y. Copper-Catalyzed Perfluoroalkylation of Alkynyl Bromides and Terminal Alkynes. Org. Lett. 2021, 23, 3190–3194; (c) Li, Z. D.; Torres-Ochoa, R. O.; Wang, Q.; Zhu, J. P. Functionalization of remote C(sp3)-H bonds enabled by copper-catalyzed coupling of O-acyloximes with terminal alkynes. Nat. Commun. 2020, 11, 403.
- 16 Wei, X. F.; Wakaki, T.; Itoh, T.; Li, H. L.; Yoshimura, T.; Miyazaki, A.; Oisaki, K.; Hatanaka, M.; Shimizu, Y.; Kanai, M. Catalytic Regio- and Enantioselective Proton Migration from Skipped Enynes to Allenes. Chem 2019, 5, 585–599.
- 17 Bakthavatchalam, R.; Ciganek, E.; Calabrese, J. C. 2,6-diarylhexahydro-4-pyridazinols by acid-catalyzed cyclization of benzaldehyde aryl-2-(2-propenyl)hydrazones. J. Heterocycl. Chem. 1996, 33, 213–216.
- 18 Feixas, J.; Prestwich, G. D.; Guerrero, A. Ligand Specificity of Pheromone-Binding Proteins of the Processionary Moth. Eur. J. Biochem. 1995, 234, 521–526.
- 19 Tajik, H.; Dadras, A.; Aghabeygi, S. A facile synthesis of novel optically active R,R-2-(4-(2-(4-(5-chloro-3-halo-pyridin-2-yloxy)-phenoxy)-propionyloxy)-phenoxy)-propionic acid esters using cyanuric chloride as potential herbicide. Chin. Chem. Lett. 2011, 22, 535–538.




