Catalytic Asymmetric [4+2] Cyclization of Hydroxyphenyl Indolinone with Azlactone to Construct Spirooxindole δ-Lactone
Comprehensive Summary
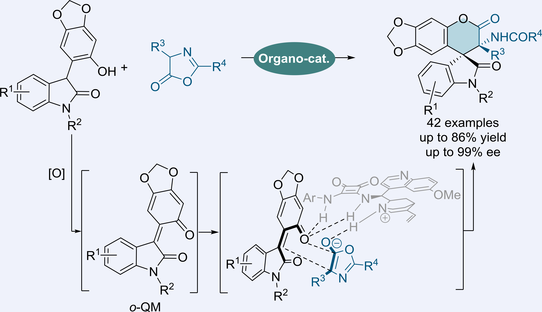
An efficient asymmetric [4+2] cyclization of hydroxyphenyl indolinone with azlactone for the synthesis of spirooxindole δ-lactone has been developed, which realized the first asymmetric reaction of hydroxyphenyl indolinone. A series of intricate structures with congested vicinal quaternary chiral centers were provided in good yields with excellent enantioselectivities via the in situ generated o-QM from hydroxyphenyl indolinone.