Molecularly Imprinted Porous-Organic Framework with pH-Responsive Adsorption Sites for the Selective Adsorption of Iron
Yajie Yang
Key Laboratory of Automobile Materials of Ministry of Education & School of Materials Science and Engineering, Jilin University, Changchun, Jilin, 130022 China
Search for more papers by this authorFuli Cai
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
Search for more papers by this authorCheng Zhang
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Nan Gao
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Suming Zhang
Beijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing, 100875 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guangtong Wang
School of Medicine and Health, Harbin Institute of Technology, Harbin, Heilongjiang, 150080 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ye Yuan
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorYajie Yang
Key Laboratory of Automobile Materials of Ministry of Education & School of Materials Science and Engineering, Jilin University, Changchun, Jilin, 130022 China
Search for more papers by this authorFuli Cai
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
Search for more papers by this authorCheng Zhang
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Nan Gao
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Suming Zhang
Beijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing, 100875 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guangtong Wang
School of Medicine and Health, Harbin Institute of Technology, Harbin, Heilongjiang, 150080 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ye Yuan
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Northeast Normal University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
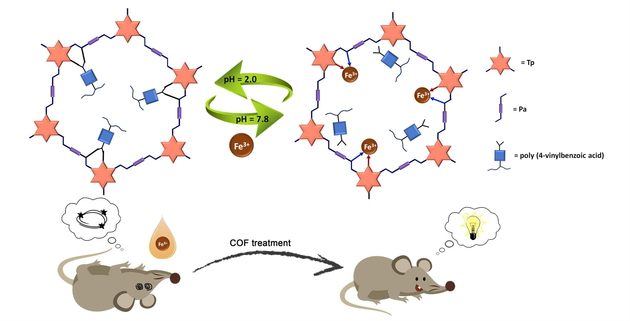
Regulating brain iron metabolism and reducing neuronal ferroptosis is proven to be a potential method for treating Alzheimer's disease (AD). However, gastric juice has a pH of 1.1—2.2 where a large number of interfering ions are dissociated from the food, which in turn causes traditional oral iron chelators to be saturated and inactivated. Herein, poly(4-vinylbenzoic acid) polymer chains were introduced as guided by Fe3+ ion template into the porous network (TpPa-1) via molecularly imprinted technology to obtain porous iron chelators, COOH@TpPa-1. The COOH@TpPa-1 maintains a multiple hydrogen bonding structure to block the channels in the stomach (pH ~1.1—2.2) with a strongly acidic environment, so just a small amount of active sites have been occupied. As COOH@TpPa-1 enters the colon, the alkaline environment disrupts the original hydrogen-bonded structure and forms anionic fragments, the bonding affinity for Fe3+ ions was ~4.0 times that in the stomach, and also gave a high selective coefficient 4.2 times higher than that of conventional iron chelators. These designable "on" and "off" states promote the effective enrichment of iron ions within the colon by the porous chelator and produce a favorable therapeutic effect on Alzheimer's symptoms caused by ferroptosis in mice.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400663-sup-0001-supinfo.pdfPDF document, 2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Kaiser, J. The Alzheimer's Gamble. Science 2018, 361, 838–841.
- 2 Ritz, B.; Yu, Y. Noise Exposure and Dementia: a Rising Concern in Ageing Populations. BMJ 2021, 374, n2120.
- 3 Spires-Jones, T. L.; Ritchie, C. W. A Brain Boost to Fight Alzheimer's Disease. Science 2018, 361, 975–976.
- 4
Lin, R.; Huang, H.; Tao, Q. Innovation Med. 2023, 1, 100020.
10.59717/j.xinn-med.2023.100020 Google Scholar
- 5 Mann, C. N.; Devi, S. S.; Kersting, C. T.; Bleem, A. V.; Karch, C. M.; Holtzman, D. M.; Gallardo, G. Astrocytic α2-Na+/K+ ATPase Inhibition Suppresses Astrocyte Reactivity and Reduces Neurodegeneration in a Tauopathy Mouse Model. Sci. Transl. Med. 2022, 14, eabm4107.
- 6 Wang, F. X.; Wang, J. D.; Shen, Y.; Li, H.; Rausch, W. D.; Huang, X. B. Iron Dyshomeostasis and Ferroptosis: A New Alzheimer's Disease Hypothesis? Front. Aging Neurosci. 2022, 14, 830569.
- 7 Baruah, P.; Moorthy, H.; Ramesh, M.; Padhi, D.; Govindaraju, T. A Natural Polyphenol Activates and Enhances GPX4 to Mitigate Amyloid-β Induced Ferroptosis in Alzheimer's Disease. Chem. Sci. 2023, 14, 9427–9438.
- 8 Wang. J.; Wang, Z.; Li, Y.; Hou, Y.; Yin, C.; Yang, E.; Liao, Z.; Fan, C.; Martin, L.; Sun, D. Blood Brain Barrier-targeted Delivery of Double Selenium Nanospheres Ameliorates Neural Ferroptosis in Alzheimer's Disease. Biomaterials 2023, 302, 122359.
- 9 Kirsch, R.; Sijtsema, H. P.; Tlali, M.; Marais, A. D.; Hall, P. Effects of Iron Overload in a Rat Nutritional Model of Non-alcoholic Fatty Liver Disease. Liver Int. 2006, 26, 1258–1267.
- 10 Pramanik, S.; Chakraborty, S.; Sivan, M.; Patro, B. S.; Chatterjee, S.; Goswami, D. Cell Permeable Imidazole-Desferrioxamine Conjugates: Synthesis and In Vitro Evaluation. Bioconjug. Chem. 2019, 30, 841–852.
- 11 Cote, A. P.; Benin, A. I.; Ockwig, N. W.; O’Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170.
- 12 Kwon, J. H.; Ma, H.; Giri, A.; Hopkins, P. E.; Shustova, N. B.; Tian, Z. T. Thermal Conductivity of Covalent-Organic Frameworks. ACS Nano 2023, 17, 15222–15230.
- 13 Wang, X.; Han, X.; Cheng, C.; Kang, X.; Liu, Y.; Cui, Y. 2D Covalent Organic Frameworks with Cem Topology. J. Am. Chem. Soc. 2022, 144, 7366–7373.
- 14 Jin, E. Q.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M. A.; Brady, M. A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q. H.; Jiang, D. L. Two-dimensional sp2 Carbon-conjugated Covalent Organic Frameworks. Science 2017, 357, 673–676.
- 15 Keller, N.; Bessinger, D.; Reuter, S.; Calik, M.; Ascherl, L.; Hanusch, F. C.; Auras, F.; Bein, T. Oligothiophene-Bridged Conjugated Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8194–8199.
- 16 Ma, J. X.; Li, J.; Chen, Y. F.; Ning, R.; Ao, Y. F.; Liu, J. M.; Sun, J. L.; Wang, D. X.; Wang, Q. Q. Cage Based Crystalline Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 3843–3848.
- 17 Yuan, Y.; Cui, P.; Tian, Y. Y.; Zou, X. Q.; Zhou, Y. X.; Sun, F. X.; Zhu, G. S. Coupling Fullerene into Porous Aromatic Frameworks for Gas Selective Sorption. Chem. Sci. 2016, 7, 3751–3756.
- 18 Yuan, Y.; Zhu, G. S. Porous Aromatic Frameworks as a Platform for Multifunctional Applications. ACS Cent. Sci. 2019, 5, 409–418.
- 19 Huang, N.; Chen, X.; Krishna, R.; Jiang, D. L. Two-dimensional Covalent Organic Frameworks for Carbon Dioxide Capture through Channel-wall Functionalization. Angew. Chem. Int. Ed. 2015, 54, 2986–2990.
- 20 Huang, N.; Krishna, R.; Jiang, D. L. Tailor-Made Pore Surface Engineering in Covalent Organic Frameworks: Systematic Functionalization for Performance Screening. J. Am. Chem. Soc. 2015, 137, 7079–7082.
- 21 Nabeela, K.; Deka, R.; Abbas, Z.; Kumar, P.; Saraf, M.; Mobin, S. M. Covalent Organic Frameworks (COFs)/MXenes Heterostructures for Electrochemical Energy Storage. Cryst. Growth Des. 2023, 23, 3057–3078.
- 22 Zhang, H. Y.; Geng, Y. B.; Huang, J.; Wang, Z. X.; Du, K.; Li, H. Y. Charge and Mass Transport Mechanisms in Two-dimensional Covalent Organic Frameworks (2D COFs) for Electrochemical Energy Storage Devices. Energy Environ. Sci. 2023, 16, 889–951.
- 23 Hao, K.; Guo, Z. P.; Lin, L.; Sun, P. J.; Li, Y. H.; Tian, H. Y.; Chen, X. S. Covalent Organic Framework Nanoparticles for Anti-tumor Gene Therapy. Sci. China Chem. 2021, 64, 1235–1241.
- 24 Lyu, X. H.; Yi, L. Z.; Zhang, L.; Liu, J.; Deng, H. X. Monodispersed Covalent Organic Framework Nanocrystals in Aqueous Solution for DNA Inclusion. Sci. China Chem. 2023, 66, 3161–3168.
- 25 He, L. W.; Li, B. Y.; Ma, Z. L.; Chen, L. X.; Gong, S. C.; Zhang, M. X.; Bai, Y. Y.; Guo, Q.; Wu, F. Q.; Zhao, F. Q.; Li, J.; Zhang, D.; Sheng, D. P.; Dai, X.; Chen, L.; Shu, J.; Chai, Z. F.; Wang, S. A. Synergy of First- and second-sphere Interactions in a Covalent Organic Framework Boosts Highly Selective Platinum Uptake. Sci. China Chem. 2023, 66, 783–790.
- 26 Zhang, M. X.; Yuan, M. J.; Zhao, X. F.; Chen, J. C.; He, L. W.; Gao, Q. H.; Hu, J. T.; Wu, G. Z.; Chai, Z. F.; Wang, S. A. Radiation-induced One-pot Synthesis of Grafted Covalent Organic Frameworks. Sci. China Chem. 2023, 66, 1781–1787.
- 27 Wang, Z. Q.; Wang, X.; Li, C.; Yang, Y. W. Covalent Organic Frameworks for Antibacterial Applications. Cell Rep. Phys. Sci. 2024, 5, 101845.
- 28 Liang, S.; Li, M. H.; Qi, M.; Wang, L.; Yang, Y. W. Nanoplatforms Based on Covalent Organic Frameworks (COFs) for Biomedical Applications. Chem. Mater. 2023, 35, 8353–8370.
- 29 Ding, S. Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W. G.; Su, C. Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki-Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822.
- 30 Gong, Y. N.; Guan, X.; Jiang, H. L. Covalent Organic Frameworks for Photocatalysis: Synthesis, Structuralfeatures, Fundamentals and Performance. Coord. Chem. Rev. 2023, 475, 214889.
- 31 Tang, Q. Q.; Gu, Y. Y.; Ning, J.; Yan, Y. K.; Shi, L.; Zhou, M. S.; Wei, H. T.; Ren, X. H.; Li, X. H.; Wang, J. X.; Tang, C.; Hao, L.; Ye, J. H. Boosting Photocatalysis of Hydrazone-linked Covalent Organic Frameworks through Introducing Electron-rich Conjugated Aldehyde. Chem. Eng. J. 2023, 470, 144106.
- 32 Li, M. H.; Xu, C.; Yang, Y. W. Macrocycle-embedded Metal-covalent Organic Frameworks for Catalysis: A Bridge Between Covalent and Non-covalent Functional Frameworks. Coord. Chem. Rev. 2024, 512, 215894.
- 33 Cui, X.; Lei, S.; Wang, A. C.; Gao, L. K.; Zhang, Q.; Yang, Y. K.; Lin, Z. Q. Emerging Covalent Organic Frameworks Tailored Materials for Electrocatalysis. Nano Energy 2020, 70, 104525.
- 34 Han, B.; Jin, Y. C.; Chen, B. T.; Zhou, W.; Yu, B. Q.; Wei, C. Y.; Wang, H. L.; Wang, K.; Chen, Y. L.; Chen, B. L.; Jiang, J. Z. Maximizing Electroactive Sites in a Three-Dimensional Covalent Organic Framework for Significantly Improved Carbon Dioxide Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202114244.
- 35 Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M. V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527.
- 36
Rebelo, S. L. H.; Neves, C. M. B.; de Almeida, M. P.; Pereira, E.; Simões, M. M. Q.; Neves, M. G. P. M. S.; de Castro, B.; Medforth, C. J. Binary Ionic Iron(III) Porphyrin Nanostructured Materials with Catalase-like Activity. Appl. Mater. Today 2020, 21, 100830.
10.1016/j.apmt.2020.100830 Google Scholar
- 37 Bartlett, A. N.; Hoffbrand, A. V.; Kontoghiorghes, G. J. Long-term Trial with the Oral Iron Chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1). II. Clinical Observations. Br. J. Haematol. 1990, 76, 301–304.
- 38 Esposito, B. P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z. I. Labile Plasma Iron in Iron Overload: Redox Activity and Susceptibility to Chelation. Blood 2003, 102, 2670–2677.




