Redox-Neutral Nickel-Catalyzed Selective Hydroalkynylation of Internal Alkyne and Its Application in Anticancer Agent Discovery†
Weiming Chen
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorTeng Liu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorShuqing Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorGuangyu Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorGaorong Wu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYoujia Gao
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorZhilin Xu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYitao Wu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorCorresponding Author
Xiaopeng Peng
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiuzhong Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWeiming Chen
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorTeng Liu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorShuqing Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
W. C., T. L. and S. L. contributed equally to this work.
Search for more papers by this authorGuangyu Li
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorGaorong Wu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYoujia Gao
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorZhilin Xu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorYitao Wu
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Search for more papers by this authorCorresponding Author
Xiaopeng Peng
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiuzhong Huang
Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, School of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, 341000 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Nickel Catalysis.
Comprehensive Summary
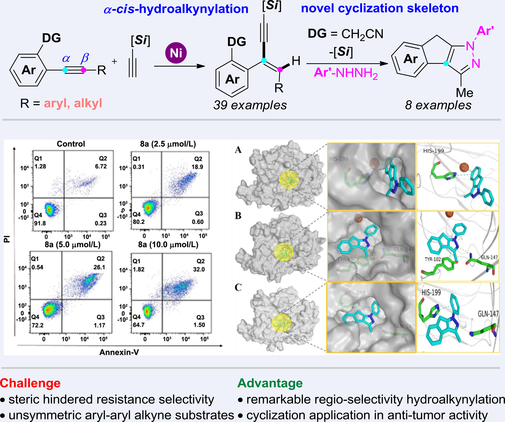
Herein, an unprecedented nickel-catalyzed regioselective hydroalkynylation of unsymmetrical internal alkynes was realized with steric hindered resistance selectivity via the cyano-directing group strategy. Significantly, the resulting 1,3-enyne products could be effectively employed in the synthesis of novel nitrogen-containing tricyclics compounds, that provided the potential candidate compound 8a (IC50 = 2.6—6.1 μmol/L) for the anti-tumor cell proliferation activity. Therefore, this work not only improves the transition-metal- catalyzed hydroalkynylation strategy of internal alkynes, but also exhibits versatility of 1,3-enynes in the construction of the complex bioactive chemical space.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400655-sup-0001-supinfo.pdfPDF document, 9.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Dherbassy, Q.; Manna, S.; Talbot, F. J.; Prasitwatcharakorn, W.; Perry, G. J.; Procter, D. J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393.
- 2 Chen, Y.; Zhu, K.; Huang, Q.; Lu, Y. Regiodivergent sulfonylarylation of 1, 3-enynes via nickel/photoredox dual catalysis. Chem. Sci. 2021, 12, 13564–13571.
- 3 Wang, Z.; Zhang, C.; Wu, J.; Li, B.; Chrostowska, A.; Karamanis, P.; Liu, S. Y. trans-Hydroalkynylation of internal 1,3-enynes enabled by cooperative catalysis. J. Am. Chem. Soc. 2023, 145, 5624–5630.
- 4 Jian, W.; Chiou, M.-F.; Li, Y.; Bao, H.; Yang, S. Cu-catalyzed regioselective diborylation of 1,3-enynes for the efficient synthesis of 1,4-diborylated allenes. Chin. Chem. Lett. 2024, 35, 108980.
- 5 Li, G. Q.; Meng, F. R.; Xiao, W. J.; Chen, J. R. Photoinduced copper-catalyzed asymmetric radical three-component cross-coupling of 1, 3-enynes with oxime esters and carboxylic acids. Org. Chem. Front. 2023, 10, 2773–2781.
- 6 Iverson, S. L.; Uetrecht, J. P. Identification of a reactive metabolite of terbinafine: insights into terbinafine-Induced Hepatotoxicity. Chem. Res. Toxicol. 2001, 14, 175–181.
- 7 Zhang, K.; Lu, L.-Q.; Yao, S.; Chen, J.-R.; Shi, D.-Q.; Xiao, W.-J. J. Am. Chem. Soc. 2017, 139, 12847–12854.
- 8 Liu, J.; Yang, J.; Schneider, C.; Franke, R.; Jackstell, R.; Beller, M. Tailored palladium catalysts for selective synthesis of conjugated enynes by monocarbonylation of 1, 3-siynes. Angew. Chem. Int. Ed. 2020, 59, 9032–9040.
- 9
Wang, Y.; Feng, J.; Li, E.; Jia, Z.; Loh, T. P. Recent advances in ligand-enabled palladium-catalyzed divergent synthesis. Org. Biomol. Chem. 2024, 22, 37–54.
10.1039/D3OB01679J Google Scholar
- 10 Trost, B. M.; Masters, J. T. Transition metal-catalyzed couplings of alkynes to 1,3-enynes: modern methods and synthetic applications. Chem. Soc. Rev. 2016, 45, 2212–2238.
- 11 Wang, Z.; Zhang, C.; Wu, J.; Li, B.; Chrostowska, A.; Karamanis, P.; Liu, S. Y. trans-Hydroalkynylation of internal 1, 3-enynes enabled by cooperative catalysis. J. Am. Chem. Soc. 2023, 145, 5624–5630.
- 12 Ji, X.; Peng, X.; Hong, H.; Xu, Y.; Nie, J.; Chen, L.; Mo, Z.; Li, Y.; Jiang, H. Expedient synthesis of dihaloalkenynes via Pd-catalyzed haloalkynylation reaction. Chem. Eur. J. 2023, 29, e202300068.
- 13 Weber, S. M.; Hilt, G. Late 3d metal-catalyzed (cross-) dimerization of terminal and internal alkynes. Front. Chem. 2021, 9, 635826.
- 14 Barsu, N.; Leutzsch, M.; Fürstner, A. Ruthenium-catalyzed trans-hydroalkynylation and trans-chloroalkynylation of internal alkynes. J. Am. Chem. Soc. 2020, 142, 18746–18752.
- 15 Ogata, K.; Atsuumi, Y.; Fukuzawa, S. I. Highly chemoselective nickel-catalyzed three-component cross-trimerization between two distinct terminal alkynes and an internal alkyne. Org. Lett. 2011, 13, 122–125.
- 16 Gorgas, N.; Stöger, B.; Veiros, L. F.; Kirchner, K. Iron(II) bis(acetylide) complexes as key intermediates in the catalytic hydrofunctionalization of terminal alkynes. ACS Catal. 2018, 8, 7973–7982.
- 17 Liang, Q.; Osten, K. M.; Song, D. Iron-catalyzed gem-specific dimerization of terminal alkynes. Angew. Chem. Int. Ed. 2017, 56, 6317–6320.
- 18 Trost, B. M.; Chan, C.; Rühter, G. Metal-mediated approach to enynes. J. Am. Chem. Soc. 1987, 109, 3486–3487.
- 19 Trost, B. M.; Sorum, M. T.; Chan, C.; Rühter, G. Palladium-catalyzed additions of terminal alkynes to acceptor alkynes. J. Am. Chem. Soc. 1997, 119, 698–708.
- 20 Ito, J.; Kitase, M.; Nishiyama, H. Cross-coupling of alkynes catalyzed by phebox-rhodium acetate complexes. Organometallics 2007, 26, 6412–6417.
- 21 Liu, M.; Tang, T.; Apolinar, O.; Matsuura, R.; Busacca, C. A.; Qu, B.; Fandrick, D. R.; Zatolochnaya, O. V.; Senanayake, C. H.; Song, J. J.; Engle, K. M. Atom-economical cross-coupling of internal and terminal alkynes to access 1,3-enynes. J. Am. Chem. Soc. 2021, 143, 3881–3888.
- 22 Chen, Z.; Nie, B.; Li, X.; Liu, T.; Li, C.; Huang, J. Ligand-controlled regiodivergent Ni-catalyzed trans-hydroboration/carboboration of internal alkynes with B2pin2. Chem. Sci. 2024, 15, 2236–2242.
- 23 Huang, J.; Yan, W.; Tan, C.; Wu, W.; Jiang, H. Palladium-catalyzed regioselective hydroboration of aryl alkenes with B2pin2. Chem. Commun. 2018, 54, 1770–1773.
- 24 Huang, J.; Ouyang, L.; Li, J.; Zheng, J.; Yan, W.; Wu, W.; Jiang, H. B2pin2-mediated palladium-catalyzed diacetoxylation of aryl alkenes with O2 as oxygen source and sole oxidant. Org. Lett. 2018, 20, 5090–5093.
- 25 Huang, J.; Li, J.; Zheng, J.; Wu, W.; Hu, W.; Ouyang, L.; Jiang, H. Dual Role of H2O2 in Palladium-Catalyzed Dioxygenation of Terminal Alkenes. Org. Lett. 2017, 19, 3354–3357.
- 26 Chen, L.; Zhang, M.; Liu, M.; Liu, Z.; Qiu, Y.; Zhang, Z.; Yu, F.; Huang, J. Rh(III)-catalyzed selective mono-and dual-functionalization/cyclizetion of 1-aryl-5-aminopyrazoles with iodonium ylides. Chem. Commun. 2024, 60, 432–435.
- 27 Tan, E.; Quinonero, O.; de Orbe, M. E.; Echavarren, A. M. Broad-Scope Rh-Catalyzed Inverse-Sonogashira Reaction Directed by Weakly Coordinating Groups. ACS Catal. 2018, 8, 2166–2172.
- 28 Zhao, W.; Lu, H. X.; Zhang, W. W.; Li, B. J. Coordination assistance: a powerful strategy for metal-catalyzed regio-and enantioselective hydroalkynylation of internal alkenes. Acc. Chem. Res. 2023, 56, 308–321.
- 29 Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Recent advances in chelation-assisted site-and stereoselective alkenyl C-H functionalization. Chem. Soc. Rev. 2021, 50, 3263–3314.
- 30 Posevins, D.; Qiu, Y.; Bäckvall, J.-E. Highly diastereoselective palladium-catalyzed oxidative carbocyclization of enallenes assisted by a weakly coordinating hydroxyl group. J. Am. Chem. Soc. 2018, 140, 3210–3214.
- 31 Ge, S.; Hartwig, J. F. Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions. J. Am. Chem. Soc. 2011, 133, 16330–16333.
- 32 Mills, L. R.; Edjoc, R. K.; Rousseaux, S. A. L. Design of an electron-withdrawing benzonitrile ligand for Ni-catalyzed cross-coupling involving tertiary nucleophiles. J. Am. Chem. Soc. 2021, 143, 10422–10428.
- 33 Dong, Z.; Xu, C.; Chang, J.; Zhou, S.; Sun, P.; Li, Y.; Chen, L.-A. Enantioselective directed nickel-catalyzed three-component reductive arylalkylation of alkenes via the carbometalation/radical cross-coupling sequence. ACS Catal. 2024, 14, 4395–4406.
- 34
Harvey, A. L. H.; MacTavish, J.; Mullins, S. J.; Proctor, G. R. Novel Synthesis of 2-Cyanoindan-1-one Enamines. J. Chem. Res. 1997, 1997, 420–421.
10.1039/a703502k Google Scholar
- 35 Rennet, P. G.; Lang, H. A.; Theresa, M. M.; John, M. S. Polycyclic Compounds. WO1997019929, 1997.
- 36 Christophe, P. Mild reaction conditions for the terminal deuteration of alkynes. Org. Lett. 2012, 14, 456–459.
- 37 Tetsuya, S.; Yu-ki, S.; Sentaro, O. Cobalt-catalyzed cross addition of silylacetylenes to internal alkynes. J. Org. Chem. 2013, 78, 3583–3591.
- 38 Ogata, K.; Sugasawa, J.; Fukuzawa, S. I. Highly chemoselective nickel-catalyzed three-component cross-trimerization of three distinct alkynes leading to 1, 3-dien-5-ynes. Angew. Chem. Int. Ed. 2009, 48, 6078–6080.
- 39 Long, J.; Zhao, R.; Cheng, G.-J.; Fang, X. Palladium-catalyzed alkyne hydrocyanation toward ligand-controlled stereodivergent synthesis of (E)- and (Z)-trisubstituted acrylonitrile. Angew. Chem. Int. Ed. 2023, 62, e202304543.
- 40 Ogata, K.; Murayama, H.; Sugasawa, J.; Suzuki, N.; Fukuzawa, S. I. Nickel-catalyzed highly regio-and stereoselective cross-trimerization between triisopropylsilylacetylene and internal alkynes leading to 1,3-diene-5-ynes. J. Am. Chem. Soc. 2009, 131, 3176–3177.
- 41 Liu, G.; Kong, W.; Che, J.; Zhu, G. Palladium-catalyzed cross addition of terminal alkynes to aryl ynamides: an unusual trans-hydroalkynylation reaction. Adv. Synth. Catal. 2014, 356, 3314–3318.
- 42 Xiao, N.; Zhan, Y. Z.; Meng, H.; Shu, W. Access to Z-selective 1,3-enynes via Ni-catalyzed intermolecular cross-alkylalkynylation of terminal alkynes. Org. Lett. 2021, 23, 5186–5191.
- 43 Liu, Y.; Wang, J.; Ji, Y.; Zhao, G.; Tang, L.; Zhang, C.; Guo, X.; Liu, Z. Design, synthesis, and biological evaluation of 1-methyl-1,4-dihydroindeno[1,2-c]pyrazole analogues as potential anticancer agents targeting tubulin colchicine binding site. J. Med. Chem. 2016, 59, 5341–5355.
- 44 Quail, D. F.; Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- 45 Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell 2008, 13, 472–482.
- 46 Kutuk, O.; Basaga, H. Bcl-2 protein family: implications in vascular apoptosis and atherosclerosis. Apoptosis 2006, 11, 1661–1675.
- 47 Fusea, S.; Suzukia, K.; Kuchimaru, T.; Kadonosono, T.; Uedaa, H.; Sato, S.; Kizaka-Kondoh, S.; Nakamura, H. Design, synthesis, and evaluation of indeno[2,1-c]pyrazolones for use as inhibitors against hypoxia-inducible factor (HIF)-1 transcriptional activity. Bioorg. Med. Chem. 2020, 28, 115207.
- 48 Giragossian, C.; Mierke, D. Intermolecular interactions between cholecystokinin-8 and the third extracellular loop of the cholecystokinin A receptor. Biochemistry 2001, 40, 3804–3809.
- 49 Dann, C.; Bruick, R.; Deisenhofer, J. Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway. Proc. Natl. Acad. Sci. 2002, 99, 15351–15356.
- 50 Hewitson, K.; Liénard, B.; McDonough, M.; Clifton, I.; Butler, D.; Soares, A.; Oldham, N.; McNeill, L.; Schofield, C. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007, 282, 3293–3301.




