Cholesterol Decorated Diazocine Gelator with Photo- and Thermo-Responsive Properties for Smart Window
Yaxin Wang
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorYuan Yuan
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShuaipeng Zhang
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorLong Chen
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Yulan Chen
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]Search for more papers by this authorYaxin Wang
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorYuan Yuan
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorShuaipeng Zhang
Key Laboratory of Organic Integrated Circuit, Ministry of Education & Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorLong Chen
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Yulan Chen
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
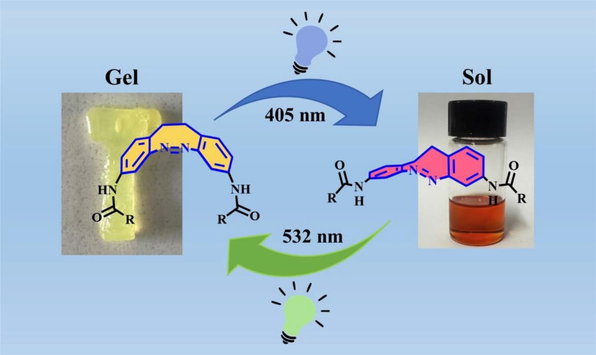
A new gelator (BAzo-Chol) comprising a diazocine core and two cholesterol units linked via carbamate groups has been designed and synthesized. Its gelation ability in several kinds of organic solvents is identified. Investigations with scanning electron microscopy (SEM), transmission electron microscopy (TEM), rheological measurements, and UV-vis absorption and 1H NMR spectroscopy reveal that BAzo-Chol can self-assemble into elastically interpenetrating one-dimensional nanostructures in organogels through a cooperative effect of the π-π interactions, hydrogen-bonding, and van der Waals forces. Moreover, the unconventional Z/E conformational transitions of these organogels, due to the inclusion of diazocine, endow the BAzo-Chol gels with excellent photo-responsive gel-sol transition and remarkable visible light-induced chromic properties. Lastly, due to its sensitive response to visible light accompanied by distinct changes in both colour and transparency of the gel-sol transitions, the potential application of the current gelator in smart windows is signified.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400527-sup-0001-supinfo.pdfPDF document, 1.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Kuzina, M.; Kartsev, D.; Stratonovich, A.; Levkin, P. Organogels Versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421.
- 2 Larik, F.; Fillbrook, L.; Nurttila, S.; Martin, A.; Kuchel, R.; Taief, K.; Bhadbhade, M.; Beves, J.; Thordarson, P. Ultra-Low Molecular Weight Photoswitchable Hydrogelators. Angew. Chem. Int. Ed. 2021, 60, 6764–6770.
- 3 Xie, T.; Yuan, W.; Li, X.; Li, M.; Chen, Y. Circularly Polarized Luminescence from Chiral p-Terphenylene-Based Supramolecular Aggregates. Chin. J. Chem. 2021, 39, 2095–2100.
- 4 Zhang, S.; Wang, X. Inorganic Subnanometer Nanowire-Based Organogels: Trends, Challenges, and Opportunities. ACS Nano 2023, 17, 20–26.
- 5 Zhang, H.; Yang, X.; Liang, J.; Chen, K.; Wang, H. Hierarchical Self-assembly of G-Quadruplexes Based Hydrogel Consisting of Guanine and Peptide Epitope. Chin. J. Chem. 2023, 41, 1727–1732.
- 6 Chen, Y.; Lv, Y.; Han, Y.; Zhu, B.; Zhang, F.; Bo, Z.; Liu, C. Dendritic Effect on Supramolecular Self-Assembly: Organogels with Strong Fluorescence Emission Induced by Aggregation. Langmuir 2009, 25, 8548–8555.
- 7 Yagai, S.; Nakajima, T.; Kishikawa, K.; Kohmoto, S.; Karatsu, T.; Kitamura, A. Hierarchical Organization of Photoresponsive Hydrogen-Bonded Rosettes. J. Am. Chem. Soc. 2005, 127, 11134–11139.
- 8 An, B.; Lee, D.; Lee, J.; Park, Y.; Song, H.; Park, S. Strongly Fluorescent Organogel System Comprising Fibrillar Self-Assembly of a Trifluoromethyl-Based Cyanostilbene Derivative. J. Am. Chem. Soc. 2004, 126, 10232–10233.
- 9
Brotin, T.; Utermohlen, R.; Fages, F.; Bouaslaurent, H.; Desvergne, J. A Novel Small Molecular Luminescent Gelling Agent for Alcohols. J. Chem. Soc. Chem. 1991, 6, 416–418.
10.1039/C39910000416 Google Scholar
- 10 Wang, M.; Nie, C.; Liu, J.; Wu, S. Organic-Inorganic Semi-Interpenetrating Networks with Orthogonal Light- and Magnetic-Responsiveness for Smart Photonic Gels. Nat. Commun. 2023, 14, 1–10.
- 11 Chai, Q.; Wei, J.; Bai, B.; Wang, H.; Li, M. A Photo-Responsive Organogel Based on Pyrene-Substituted Acylhydrazone Derivative. Chin. J. Chem. 2017, 35, 1829–1834.
- 12 Yu, X.; Chen, H.; Shi, X.; Albouy, P.; Guo, J.; Hu, J.; Li, M. Liquid Crystal Gelators with Photo-Responsive and AIE Properties. Mater. Chem. Front. 2018, 2, 2245–2253.
- 13 Das, B.; Pramanik, B.; Chowdhuri, S.; Scherman, O.; Das, D. Light- Triggered Syneresis of a Water Insoluble Peptide-Hydrogel Effectively Removes Small Molecule Waste Contaminants. Chem. Commun. 2020, 56, 3393–3396.
- 14 Meeks, A.; Lerch, M.; Schroeder, T.; Shastri, A.; Aizenberg, J. Spiropyran Photoisomerization Dynamics in Multiresponsive Hydrogels. J. Am. Chem. Soc. 2022, 144, 219–227.
- 15 Vidavsky, Y.; Yang, S.; Abel, B.; Agami, I.; Diesendruck, C.; Coates, G.; Silberstein, M. Enabling Room-Temperature Mechanochromic Activation in a Glassy Polymer: Synthesis and Characterization of Spiropyran Polycarbonate. J. Am. Chem. Soc. 2019, 141, 10060–10067.
- 16 He, W.; Yuan, Y.; Wu, M.; Li, X.; Shen, Y.; Qu, Z.; Chen, Y. Multicolor Chromism from a Single Chromophore through Synergistic Coupling of Mechanochromic and Photochromic Subunits. Angew. Chem. Int. Ed. 2023, 62, e202218785.
- 17 Wang, Y.; Li, M.; Yan, C.; Ma, N.; Chen, Y. Diazocine as a Versatile Building Block Enables Excellent Photoswitching and Chromic Properties in Self-Assembled Organogels. CCS Chem. 2022, 4, 704–712.
- 18 Wu, Y.; Wu, S.; Tian, X.; Wang, X.; Wu, W.; Zou, G.; Zhang, Q. Photoinduced Reversible Gel-Sol Transitions of Dicholesterol-Linked Azobenzene Derivatives through Breaking and Reforming of Van Der Waals Interactions. Soft Matter. 2011, 7, 716–721.
- 19 Bandarab, D.; Burdette, S. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825.
- 20 Siewertsen, R.; Neumann, H.; Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly Efficient Reversible Z-E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(nπ*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595.
- 21 Ewert, J.; Heintze, L.; Jorda-Redondo, M.; Glasenapp, J.; Nonell, S.; Bucher, G.; Peifer, C.; Herges, R. Photoswitchable Diazocine-Based Estrogen Receptor Agonists: Stabilization of the Active Form inside the Receptor. J. Am. Chem. Soc. 2022, 144, 15059–15071.
- 22 Hammerich, M.; Schutt, C.; Stahler, C.; Lentes, P.; Rohricht, F.; Hoppner, R.; Herges, R. Heterodiazocines: Synthesis and Photochromic Properties, trans to cis Switching within the Bio-optical Window. J. Am. Chem. Soc. 2016, 138, 13111–13114.
- 23 Lentes, P.; Stadler, E.; Rohricht, F.; Brahms, A.; Grobner, J.; Sonnichsen, F. D.; Gescheidt, G.; Herges, R. Nitrogen Bridged Diazocines: Photochromes Switching within the Near-Infrared Region with High Quantum Yields in Organic Solvents and in Water. J. Am. Chem. Soc. 2019, 141, 13592–13600.
- 24 Maier, M.; Hull, K.; Reynders, M.; Matsuura, B.; Leippe, P.; Ko, T.; Schaffer, L.; Trauner, D. Oxidative Approach Enables Efficient Access to Cyclic Azobenzenes. J. Am. Chem. Soc. 2019, 141, 17295–17304.
- 25 Li, S.; Eleya, N.; Staubitz, A. Cross-Coupling Strategy for the Synthesis of Diazocines. Org. Lett. 2020, 22, 1624–1627.
- 26 Shen, X.; Zhang, C.; Lan, F.; Su, Z.; Zheng, Y.; Zheng, T.; Xiong, Q.; Xie, X.; Du, G.; Zhao, X.; Hu, C.; Deng, P.; Yu, Z. Dibenzo[b,f][1,4,5]chalcogenadiazepine Photoswitches: Conversion of Excitation Energy into Ring Strain. Angew. Chem. Int. Ed. 2022, 61, e202209441.
- 27
Qiang, L.; Bai, H.; Li, X.; Yang, H.; Gong, C.; Tang, Q. A Visible Light Responsive Smart Covalent Organic Framework with a Bridged Azobenzene Backbone. Macromol. Rapid. Commun. 2023, 45, e2300506.
10.1002/marc.202300506 Google Scholar
- 28 Goual, N.; Maisonneuve, S.; Retailleau, P.; Xie, J.; Marinetti, A.; Voituriez, A. Synthesis and Characterization of a [1,2,6]Diazaphosphonine Oxide: An Example of a Photoswitchable Phosphorus-Containing Cyclic Azobenzene. J. Org. Chem. 2024, 89, 5098–5103.
- 29 Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Azoheteroarene and Diazocine Molecular Photoswitches: Self-Assembly, Responsive Materials and Photopharmacology. J. Am. Chem. Soc. 2009, 131, 15594–15595.
- 30 Lee, H.; Tessarolo, J.; Langbehn, D.; Baksi, A.; Herges, R.; Clever, G. H. Light-Powered Dissipative Assembly of Diazocine Coordination Cages. J. Am. Chem. Soc. 2022, 144, 3099–3105.
- 31 Hugenbusch, D.; Lehr, M.; von Glasenapp, J.; McConnell, A.; Herges, R. Light-Controlled Destruction and Assembly: Switching between Two Differently Composed Cage-Type Complexes. Angew. Chem. Int. Ed. 2022, 62, e202212571.
- 32 Liu, G.; Sheng, J.; Teo, W. L.; Yang, G.; Wu, H.; Li, Y.; Zhao, Y. Control on Dimensions and Supramolecular Chirality of Self-Assemblies through Light and Metal Ions. J. Am. Chem. Soc. 2018, 140, 16275–16283.
- 33 Sell, H.; Nather, C.; Herges, R. Amino-Substituted Diazocines as Pincer-Type Photochromic Switches. Beilstein J. Org. Chem. 2013, 9, 1–7.
- 34 Kim, H.; Yang, S. Responsive Smart Windows from Nanoparticle- Polymer Composites. Adv. Funct. Mater. 2020, 30, 1902597.
- 35 Meng, W.; Gao, Y.; Hu, X.; Tan, L.; Li, L.; Zhou, G.; Yang, H.; Wang, J.; Jiang, L. Photothermal Dual Passively Driven Liquid Crystal Smart Window. ACS Appl. Mater. Interfaces 2022, 14, 28301–28309.
- 36 Xia, Y.; Liang, X.; Jiang, Y.; Wang, S.; Qi, Y.; Liu, Y.; Yu, L.; Yang, H.; Zhao, X. High-Efficiency and Reliable Smart Photovoltaic Windows Enabled by Multiresponsive Liquid Crystal Composite Films and Semi-Transparent Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900720.




