Substituent Modulated Electronic Properties of Cu(I) Active Site in Metal–Organic Halides for Boosting Hydrogen Evolution Reaction†
Jing Wu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
These authors contributed equally to this work.
Search for more papers by this authorPingping Wang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
These authors contributed equally to this work.
Search for more papers by this authorYuzhe Fu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorYi Shen
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorBin Wang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorFeng Hu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorMengkai Zuo
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Wei Huang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dayu Wu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJing Wu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
These authors contributed equally to this work.
Search for more papers by this authorPingping Wang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
These authors contributed equally to this work.
Search for more papers by this authorYuzhe Fu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorYi Shen
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorBin Wang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorFeng Hu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorMengkai Zuo
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Wei Huang
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dayu Wu
Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis & Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
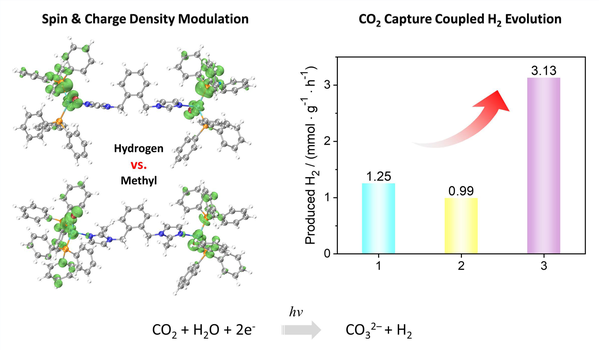
Development of heterogeneous molecular photocatalysts for promising light-driven hydrogen evolution reaction (HER) is highly demanding but still challenging. Here, we report the blue-greenish emitting dinuclear metal–organic halides as photocatalyst by incorporating site-specific single copper(I) atoms that exhibit an efficient carbon-negative H2 production. Interestingly, the electronic properties, including the spin and charge density of central Cu(I) active site, can be triggered by substituent modulation in metal–organic halides, which greatly affect the exciton dissociation kinetics and thus the HER reactivity. The optimized spin density in these heterogeneous photocatalysts drastically boosts the hydrogen production rate from 1250 to 3130 μmol·g–1·h–1. Our molecular strategy provides a platform that rationally facilitates electronic modulation of copper(I) atoms, tunes the macroscopic optoelectronic properties of photocatalysts and boosts carbon-negative HER activity, extending the boundaries of conventional molecular-based photocatalysts.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400514-sup-0001-supinfo.pdfPDF document, 2.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Wang, L.; Xiao, H.; Cheng, T.; Li, Y.; Goddard, W. A. Pb-Activated Amine-Assisted Photocatalytic Hydrogen Evolution Reaction on Organic–Inorganic Perovskites. J. Am. Chem. Soc. 2018, 140, 1994–1997.
- 2 Pi, Y.; Feng, X.; Song, Y.; Xu, Z.; Li, Z.; Lin, W. Metal–Organic Frameworks Integrate Cu Photosensitizers and Secondary Building Unit- Supported Fe Catalysts for Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2020, 142, 10302–10307.
- 3 Zhou, C.; Shi, R.; Waterhouse, G. I. N.; Zhang, T. Recent Advances in Niobium-Based Semiconductors for Solar Hydrogen Production. Coord. Chem. Rev. 2020, 419, 213399.
- 4 Zee, D. Z.; Chantarojsiri, T.; Long, J. R.; Chang, C. J. Metal–Polypyridyl Catalysts for Electro- and Photochemical Reduction of Water to Hydrogen. Acc. Chem. Res. 2015, 48, 2027–2036.
- 5 Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535.
- 6 Wu, J.; Zhou, C.; Zhao, Y.; Shang, L.; Bian, T.; Shao, L.; Shi, F.; Wu, L.; Tung, C.; Zhang, T. One-Pot Hydrothermal Synthesis and Photocatalytic Hydrogen Evolution of Pyrochlore Type K2Nb2O6. Chin. J. Chem. 2014, 32, 485–490.
- 7 Zhang, T.; Lin, W. Metal–Organic Frameworks for Artificial Photosynthesis and Photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993.
- 8 Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947.
- 9 Yang, Y.; Zhou, C.; Wang, W.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Song, B.; Xue, W.; Li, X.; Wang, Z.; He, D.; Luo, H.; Ouyang, Z. Recent Advances in Application of Transition Metal Phosphides for Photocatalytic Hydrogen Production. Chem. Eng. J. 2021, 405, 126547.
- 10 Zhao, H.; Jiang, Z.; Xiao, K.; Sun, H.; Chan, H. S.; Tsang, T. H.; Yang, S.; Wong, P. K. Photo-Assisted Separation of Noble-Metal-Free Oxidation and Reduction Cocatalysts for Graphitic Carbon Nitride Nanosheets with Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2021, 280, 119456.
- 11
Xiao, M.; Zhang, L.; Luo, B.; Lyu, M.; Wang, Z.; Huang, H.; Wang, S.; Du, A.; Wang, L. Molten–Salt–Mediated Synthesis of an Atomic Nickel Co–catalyst on TiO2 for Improved Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. 2020, 132, 7297–7301.
10.1002/ange.202001148 Google Scholar
- 12 Liu, L.; Du, S.; Guo, X.; Xiao, Y.; Yin, Z.; Yang, N.; Bao, Y.; Zhu, X.; Jin, S.; Feng, Z.; Zhang, F. Water-Stable Nickel Metal–Organic Framework Nanobelts for Cocatalyst–Free Photocatalytic Water Splitting to Produce Hydrogen. J. Am. Chem. Soc. 2022, 144, 2747–2754.
- 13 Wu, G.; Mo, Z.; Sun, P.; Cao, Z.; Zhu, X.; Song, Y.; Wei, Y.; She, X.; Li, H.; Xu, H. Improved Atomic Hydrogen Desorption by Cu3N with Suitable Electronic Structure to Enhance Photocatalytic H2 Evolution. Mater. Today Energy 2022, 29, 101111.
- 14 Hockin, B. M.; Li, C.; Robertson, N.; Zysman-Colman, E. Photoredox Catalysts Based on Earth–Abundant Metal Complexes. Catal. Sci. Technol. 2019, 9, 889–915.
- 15 Guan, X.; Qian, Y.; Zhang, X.; Jiang, H. Enaminone–Linked Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2023, 62, e202306135.
- 16 Chang, K.; Hai, X.; Ye, J. Transition Metal Disulfides as Noble–Metal–Alternative Co–Catalysts for Solar Hydrogen Production. Adv. Energy Mater. 2016, 6, 1502555.
- 17 Zhang, C.; Xie, C.; Gao, Y.; Tao, X.; Ding, C.; Fan, F.; Jiang, H. Charge Separation by Creating Band Bending in Metal–Organic Frameworks for Improved Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202204108.
- 18 Guo, F.; Guo, J. H.; Wang, P.; Kang, Y. S.; Liu, Y.; Zhao, J.; Sun, W. Y. Facet–Dependent Photocatalytic Hydrogen Production of Metal–Organic Framework NH2–MIL–125(Ti). Chem. Sci. 2019, 10, 4834–4838.
- 19 Esswein, A. J.; Nocera, D. G. Hydrogen Production by Molecular Photocatalysis. Chem. Rev. 2007, 107, 4022–4047.
- 20 Li, L.; Zhu, S.; Hao, R.; Wang, J.-J.; Yang, E.-C.; Zhao, X.-J. Amino Group Promoted Photocatalytic Hydrogen Evolution Activity Observed in Two Copper(II)-Based Layered Complexes. Dalton Trans. 2018, 47, 12726–12733.
- 21 Cao, Y.; Yin, D.; Li, S.; Dong, X.; Feng, Y.; Liu, H.; Fan, L.; Gao, G.; Zang, S. Substituent Effect to Fine–Tune Energy Levels of Atom–Precise [MoOS3]2− Modified Copper(I) Thiolate Clusters Boosting Recyclable Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202307678.
- 22 Chen, Z.; Wang, T.; Sun, T.; Chen, Z.; Sheng, T.; Hong, Y.; Nan, Z.; Zhu, J.; Zhou, Z.; Xia, H.; Sun, S. Nickel Complexes with Non-innocent Ligands as Highly Active Electrocatalysts for Hydrogen Evolution. Chin. J. Chem. 2018, 36, 1161–1164.
- 23 Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal–Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 1600371.
- 24 Shi, Y.; Yang, A. F.; Cao, C. S.; Zhao, B. Applications of MOFs: Recent Advances in Photocatalytic Hydrogen Production from Water. Coord. Chem. Rev. 2019, 390, 50–75.
- 25 Wang, Z. D.; Zang, Y.; Liu, Z. J.; Peng, P.; Wang, R.; Zang, S. Q. Opening Catalytic Sites in the Copper-Triazoles Framework via Defect Chemistry for Switching on the Proton Reduction. Appl. Catal. B Environ. 2021, 288, 119941.
- 26 Jing, X.; He, C.; Yang, Y.; Duan, C. A Metal–Organic Tetrahedron as a Redox Vehicle to Encapsulate Organic Dyes for Photocatalytic Proton Reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974.
- 27 Zhang, H.; Hong, Q.; Li, J.; Wang, F.; Huang, X.; Chen, S.; Tu, W.; Yu, D.; Xu, R.; Zhou, T.; Zhang, J. Isolated Square–Planar Copper Center in Boron Imidazolate Nanocages for Photocatalytic Reduction of CO2 to CO. Angew. Chem. Int. Ed. 2019, 58, 11752–11756.
- 28 Barlow, J. M.; Yang, J. Y. Thermodynamic Considerations for Optimizing Selective CO2 Reduction by Molecular Catalysts. ACS Cent. Sci. 2019, 5, 580–588.
- 29 Wagner, A.; Sahm, C. D.; Reisner, E. Towards Molecular Understanding of Local Chemical Environment Effects in Electro- and Photocatalytic CO2 Reduction. Nat. Catal. 2020, 3, 775–786.
- 30 Zhang, M. T.; Chen, Z.; Kang, P.; Meyer, T. J. Electrocatalytic Water Oxidation with a Copper(II) Polypeptide Complex. J. Am. Chem. Soc. 2013, 135, 2048–2051.
- 31 Zhang, T.; Wang, C.; Liu, S.; Wang, J.-L.; Lin, W. A Biomimetic Copper Water Oxidation Catalyst with Low Overpotential. J. Am. Chem. Soc. 2014, 136, 273–281.
- 32 Shi, D.; Zheng, R.; Sun, M.; Cao, X.; Sun, C.; Cui, C.; Liu, C.; Zhao, J.; Du, M. Semiconductive Copper(I)–Organic Frameworks for Efficient Light–Driven Hydrogen Generation Without Additional Photosensitizers and Cocatalysts. Angew. Chem. Int. Ed. 2017, 56, 14637–14641.
- 33 Akinaga, Y.; Kawawaki, T.; Kameko, H.; Yamazaki, Y.; Yamazaki, K.; Nakayasu, Y.; Kato, K.; Tanaka, Y.; Hanindriyo, A. T.; Takagi, M.; Shimazaki, T.; Tachikawa, M.; Yamakata, A.; Negishi, Y. Metal Single–Atom Cocatalyst on Carbon Nitride for the Photocatalytic Hydrogen Evolution Reaction: Effects of Metal Species. Adv. Funct. Mater. 2023, 33, 2303321.
- 34 Huang, N. Y.; He, H.; Li, H.; Liao, P.-Q.; Chen, X. M. A Metal–Organic Framework with in Situ Generated Low–Coordinate Binuclear Cu(I) Units as a Highly Effective Catalyst for Photodriven Hydrogen Production. Chem. Commun. 2020, 56, 6700–6703.
- 35 Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; Ma, J.; Lin, L.; Tang, J.; Huang, R.; Liu, Q. Single–Atom Cu Anchored Catalysts for Photocatalytic Renewable H2 Production with a Quantum Efficiency of 56%. Nat. Commun. 2022, 13, 58.
- 36 Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733.
- 37 Steckler, T. T.; Henriksson, P.; Mollinger, S.; Lundin, A.; Salleo, A.; Andersson, M. R. Very Low Band Gap Thiadiazoloquinoxaline Donor–Acceptor Polymers as Multi–Tool Conjugated Polymers. J. Am. Chem. Soc. 2014, 136, 1190–1193.
- 38 Song, X.; Zhang, Y.; Li, Y.; Li, F.; Bao, X.; Ding, D.; Sun, M.; Yang, R. Fluorene Side–Chained Benzodithiophene Polymers for Low Energy Loss Solar Cells. Macromolecules 2017, 50, 6880–6887.
- 39 Damas, G.; Marchiori, C. F. N.; Araujo, C. M. On the Design of Donor–Acceptor Conjugated Polymers for Photocatalytic Hydrogen Evolution Reaction: First–Principles Theory–Based Assessment. J. Phys. Chem. C 2018, 122, 26876–26888.
- 40 Dong, X.; Zhang, M.; Pei, R.; Wang, Q.; Wei, D.; Zang, S.; Fan, Y.; Mak, T. C. W. A Crystalline Copper(II) Coordination Polymer for the Efficient Visible–Light–Driven Generation of Hydrogen. Angew. Chem. Int. Ed. 2016, 55, 2073–2077.
- 41
Wu, Z.; Wang, C.; Zhao, B.; Dong, J.; Lu, F.; Wang, W.; Wang, W.; Wu, G.; Cui, J.; Cheng, P. A Semi–Conductive Copper–Organic Framework with Two Types of Photocatalytic Activity. Angew. Chem. Int. Ed. 2016, 128, 5022–5026.
10.1002/ange.201508325 Google Scholar
- 42 Cao, Y. D.; Hao, H. P.; Liu, H. S.; Yin, D.; Wang, M. L.; Gao, G. G.; Fan, L. L.; Liu, H. A 20-Core Copper(I) Nanocluster as Electron–Hole Recombination Inhibitor on TiO2 Nanosheets for Enhancing Photocatalytic H2 Evolution. Nanoscale 2021, 13, 16182–16188.
- 43 Xu, L.; Yang, C.; Cheng, Z.; Huang, Q.; Zhang, S.; Qian, C.; Liao, Y. Carbazole–Based Donor–Acceptor Conjugated Microporous Polymers for Efficient Visible–Light–Driven Photocatalytic H2 Evolution. Chin. J. Chem. 2023, 41, 2518–2524.
- 44 Liu, N.; Jiang, J.; Chen, Z.; Wu, B.; Zhang, S.; Zhang, Y.; Cheng, P.; Shi, W. Promoted Photocatalytic Hydrogen Evolution by Tuning the Electronic State of Copper Sites in Metal–Organic Supramolecular Assemblies. Angew. Chem. Int. Ed. 2023, 62, e202312306.
- 45 Keith, D. W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594.
- 46 Gao, M.; Fan, J.; Li, X.; Wang, Q.; Li, D.; Feng, J.; Duan, X. A Carbon-Negative Hydrogen Production Strategy: CO2 Selective Capture with H2 Production. Angew. Chem. Int. Ed. 2023, 62, e202216527.
- 47 Zhu, X.; Xie, W.; Wu, J.; Miao, Y.; Xiang, C.; Chen, C.; Ge, B.; Gan, Z.; Yang, F.; Zhang, M.; O’Hare, D.; Li, J.; Ge, T.; Wang, R. Recent Advances in Direct Air Capture by Adsorption. Chem. Soc. Rev. 2022, 51, 6574–6651.
- 48Deposition Numbers 2350233—2350235 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 49 Zhou, H.; Chen, R.; Han, C.; Wang, P.; Tong, Z.; Tan, B.; Huang, Y.; Liu, Z. Copper Phosphide Decorated G-C3N4 Catalysts for Highly Efficient Photocatalytic H2 Evolution. J. Colloid Interface Sci. 2022, 610, 126–135.
- 50 Li, M.; Cheng, Z.; Wang, X.; Yu, Z.; Zhou, M.; Miao, H.; Zhaxi, W.; Huang, W.; Ma, X.; Chen, Q.; Jiang, S.; Zhang, Q.; Wu, D. Negative/Zero Thermal Quenching of Luminescence via Electronic Structural Transition in Copper–Iodide Cluster-Based Coordination Networks. J. Phys. Chem. Lett. 2021, 12, 8237–8245.
- 51 Wang, P.; Miao, H.; Sheng, K.; Wang, B.; Feng, F.; Cai, X.; Huang, W.; Wu, D. Efficient Blue-Light-Excitable Copper(I) Coordination Network Phosphors for High–Performance White LEDs. Chin. Chem. Lett. 2024, 35, 108600.
- 52 Hill, I. M.; Hanspal, S.; Young, Z. D.; Davis, R. J. DRIFTS of Probe Molecules Adsorbed on Magnesia, Zirconia, and Hydroxyapatite Catalysts. J. Phys. Chem. C 2015, 119, 9186–9195.
- 53 Su, B.; Zheng, M.; Lin, W.; Lu, X. F.; Luan, D.; Wang, S.; Lou, X. W. S-Scheme Co9S8@Cd0.8Zn0.2S-DETA Hierarchical Nanocages Bearing Organic CO2 Activators for Photocatalytic Syngas Production. Adv. Energy Mater. 2023, 13, 2203290.
- 54 Su, B.; Kong, Y.; Wang, S.; Zuo, S.; Lin, W.; Fang, Y.; Hou, Y.; Zhang, G.; Zhang, H.; Wang, X. Hydroxyl-Bonded Ru on Metallic TiN Surface Catalyzing CO2 Reduction with H2O by Infrared Light. J. Am. Chem. Soc. 2023, 145, 27415–27423.
- 55 Skulason, E.; Tripkovic, V.; Bjorketun, M. E.; Gudmundsdottir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jonsson, H.; Norskov, J. K. Modeling the Electrochemical Hydrogen Oxidation and Evolution Reactions on the Basis of Density Functional Theory Calculations. J. Phys. Chem. C 2010, 114, 18182–18197.
- 56 Gao, G.; O'Mullane, A. P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500.
- 57 Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; et al. Gaussian 16, Rev. B.01, Wallingford, CT, 2016.
- 58 Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. Ab Initio Calculation of Vibrational Absorption and Circular-Dichroism Spectra Using Density-Functional Force-Fields. J. Phys. Chem. 1994, 98, 11623–11627.
- 59 Becke, A. D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652.
- 60 Rassolov, V. A.; Ratner, M. A.; Pople, J. A.; Redfern, P. C.; Curtiss, L. A. 6-31G* Basis Set for Third-Row Atoms. J. Comput. Chem. 2001, 22, 976–984.
- 61 Szentpaly, L. V.; Fuentealba, P.; Preuss, H.; Stoll, H. Pseudopotential Calculations on Rb+2, Cs+2, RbH+, CsH+ and the Mixed Alkali Dimer Ions. Chem. Phys. Lett. 1982, 93, 555–559.
- 62 Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38.
- 63 Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592.
- 64 Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186.
- 65 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
- 66 Hammer, B.; Hansen, L. B.; Norskov, J. K. Improved Adsorption Energetics within Density-Functional Theory Using Revised Perdew- Burke-Ernzerhof Functionals. Phys. Rev. B 1999, 59, 7413–7421.
- 67 Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799.
- 68 Skulason, E.; Tripkovic, V.; Bjorketun, M. E.; Gudmundsdottir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jonsson, H.; Nørskov, J. K. Modeling the Electrochemical Hydrogen Oxidation and Evolution Reactions on the Basis of Density Functional Theory Calculations. J. Phys. Chem. C 2010, 114, 18182–18197.
- 69 Gao, G.; O'Mullane, A. P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500.




