Versatile Synthesis of α-Oxygen Organoboron Compounds via Photo-Induced Siloxycarbene†
Xiongxiong Lu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
The authors contributed equally to this work.
Search for more papers by this authorQingbin Zhao
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
The authors contributed equally to this work.
Search for more papers by this authorDehai Cao
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorPan Xu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorXuenian Chen
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Henan Key Laboratory of Boron Chemistry and Advanced Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Zhenxing Liu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorXiongxiong Lu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
The authors contributed equally to this work.
Search for more papers by this authorQingbin Zhao
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
The authors contributed equally to this work.
Search for more papers by this authorDehai Cao
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorPan Xu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorXuenian Chen
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Henan Key Laboratory of Boron Chemistry and Advanced Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Zhenxing Liu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Boron Chemistry.
Comprehensive Summary
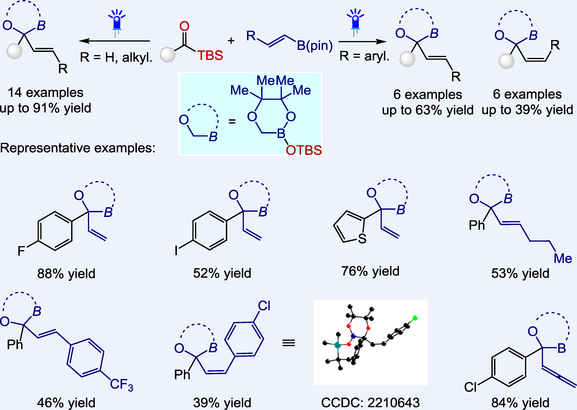
A novel method for synthesizing α-oxygen organoboron compounds has been developed through acylsilane-based carbene insertion reactions into C—B bonds. As coupling partners, readily available organoboron compounds (alkenyl, allyl, and allenyl B(pin)) were employed. Based on the substrates, pure insertion into C—B bonds or insertion followed by a siloxy group rearrangement process (from carbon to boron) would occur, delivering the α-oxygen organoboron compounds with great diversities. Control experiments demonstrated that the electronic effect of the substituents mainly controlled the rearrangement process. Besides, no matter which isomer of substrate (Z or E) was used, the reaction with β-aryl-substituted alkenyl B(pin) affords both isomers of products (Z and E, separable through column chromatography). Trapping experiments indicated the triplet energy transfer process was involved.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400497-sup-0001-supinfo.pdfPDF document, 7.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Brown, H. C.; Zweifel, G. A stereospecific cis hydration of the double bond in cyclic derivatives. J. Am. Chem. Soc. 1959, 81, 247.
- 2 Brown, H. C. Hydroboration-a powerful synthetic tool. Tetrahedron 1961, 12, 117.
- 3 Miyaura, N.; Yamada K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or l-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440.
- 4 Miyaura, N.; Yamada, K.; Suginome, H.; Suzuki, A. Novel and convenient method for the stereo- and regiospecific synthesis of conjugated alkadienes and alkenynes via the palladium-catalyzed cross-coupling reaction of 1-alkenylboranes with bromoalkenes and bromoalkynes. J. Am. Chem. Soc. 1985, 107, 972–980.
- 5 Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483.
- 6 Yang, W.; Gao, X.; Wang, B. Boronic acid compounds as potential pharmaceutical agents. Med. Res. Rev. 2003, 23, 346–368.
- 7 Milo, L. J. Jr.; Lai, J. H.; Wu, W.; Liu, Y.; Maw, H.; Li, Y.; Jin, Z.; Shu, Y.; Poplawski, S. E.; Wu, Y.; Sanford, D. G.; Sudmeier, J. L.; Bachovchin, W. W. Chemical and biological evaluation of dipeptidyl boronic acid proteasome inhibitors for use in prodrugs and pro-soft drugs targeting solid tumors. J. Med. Chem. 2011, 54, 4365–4377.
- 8 Dembitsky, V. M.; Al Quntar, A. A. A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 209–237.
- 9
Entwistle, C. D.; Marder, T. B. Boron chemistry lights the way: optical properties of molecular and polymeric systems. Angew. Chem. Int. Ed. 2002, 41, 2927–2931.
10.1002/1521-3773(20020816)41:16<2927::AID-ANIE2927>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 10 Jäkle, F. Advances in the synthesis of organoborane polymers for optical, electronic, and sensory applications. Chem. Rev. 2010, 110, 3985–4022.
- 11 Ji, L.; Griesbeck, S.; Marder, T. B. Recent developments in and perspectives on three-coordinate boron materials: a bright future. Chem. Sci. 2017, 8, 846–863.
- 12 Ramachandran, P. V. Pinane-based versatile “allyl”boranes. Aldrichim. Acta 2002, 35, 23–25.
- 13 Diner, C.; Szabó, K. J. Recent advances in the preparation and application of allylboron species in organic synthesis. J. Am. Chem. Soc. 2017, 139, 2–14.
- 14 Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P. J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericàs, M. A.; Diéguez, M. Recent advances in enantioselective Pd-catalyzed allylic substitution: from design to applications. Chem. Rev. 2021, 121, 4373–4505.
- 15 Miyarura, N. Organoboron compounds. Top. Curr. Chem. 2002, 219, 11–59.
- 16 Brook, A. G. Triphenylsilyl phenyl ketone. J. Am. Chem. Soc. 1957, 79, 43734375.
- 17 Page, P. C. B.; Klair, S. S.; Rosenthal, S. Synthesis and chemistry of acyl silanes. Chem. Soc. Rev. 1990, 19, 147–195.
- 18 Zhang, H.-J.; Priebbenow, D. L.; Bolm, C. Acylsilanes: valuable organosilicon reagents in organic synthesis. Chem. Soc. Rev. 2013, 42, 8540–8571.
- 19 Wang, X.; Liu, F.; Li, Y.; Yan, Z.; Qiang, Q.; Rong, Z.-Q. Recent advances in the synthesis of acylsilanes. ChemCatChem 2020, 12, 5022–5233.
- 20 Holownia, A.; Apte, C. N.; Yudin, A. K. Acyl metalloids: conformity and deviation from carbonyl reactivity. Chem. Sci. 2021, 12, 5346–5360.
- 21 Hong, W. P.; Lim, H. N.; Shin, I. Recent progress and perspectives in photo-induced organic reactions of acylsilanes. Org. Chem. Front. 2023, 10, 819–836.
- 22 Zhao, Q.; Geng, Q.; Li, Y.; Li, J.; Liu, Z. Emerging applications of acylsilanes in organic synthesis and beyond. Org. Chem. Front. 2023, 10, 1316–1321.
- 23 Zhou, G.; Guo, Z.; Shen, X. Electron-rich oxycarbenes: new synthetic and catalytic applications beyond group 6 Fischer carbene complexes. Angew. Chem. Int. Ed. 2023, 62, e202217189.
- 24 Ishida, K.; Tobita, F.; Kusama, H. Lewis acid-assisted photo-induced intermolecular coupling between acylsilanes and aldehydes: a formal cross benzoin type condensation. Chem. Eur. J. 2018, 24, 543–546.
- 25 Ma, L.; Yu, Y.; Xin, L.; Zhu, L.; Xia, J.; Ou, P.; Huang, X. Visible light enabled formal cross silyl benzoin reaction as an access to α-hydroxyketones. Adv. Synth. Catal. 2021, 363, 2573–2577.
- 26 Fan, Z.; Yi, Y.; Chen, S.; Xi, C. Visible-light-induced catalyst-free carboxylation of acylsilanes with carbon dioxide. Org. Lett. 2021, 23, 2303–2307.
- 27 Priebbenow, D. L.; Pilkington, R. L.; Hearn, K. N.; Polyzos, A. Fluorinated ketones as trapping reagents for visible-light-induced singlet nucleophilic carbenes. Org. Lett. 2021, 23, 2783–2789.
- 28 Becker, P.; Priebbenow, D. L.; Zhang, H.-J.; Pirwerdjan, R.; Bolm, C. Photochemical intermolecular silylacylations of electron-deficient internal alkynes. J. Org. Chem. 2014, 79, 814–817.
- 29(a) Zhou, G.; Shen, X. Synthesis of cyclopropenols enabled by visible-light-induced organocatalyzed [2+1] cyclization. Angew. Chem. Int. Ed. 2022, 61, e202115334; (b) Zhang, Y.; Zhou, G.; Gong, X.; Guo, Z.; Qi, X.; Shen, X. Diastereoselective transfer of tri(di)fluoroacetylsilanes-derived carbenes to alkenes. Angew. Chem. Int. Ed. 2022, 61, e202202175; (c) Zhang, Y.; Zhou, G.; Gong, X.; Guo, Z.; Qi, X.; Shen, X. Diastereoselective transfer of tri(di)fluoroacetylsilanes-derived carbenes to alkenes. Angew. Chem. Int. Ed. 2022, 61, e202202175.
- 30(a) Stuckhardt, C.; Wissing, M.; Studer, A. Photo Click Reaction of Acylsilanes with Indoles. Angew. Chem. Int. Ed. 2021, 60, 18605–18611; (b) Lind, F.; Studer, A. Rhodium-Catalyzed Hydrosilylation of Alkenes with Acylhydrosilanes. Chem. Eur. J. 2023, 29, e202301120; (c) Lind, F.; Markelov, K.; Studer, A. Benzoyldiisopropylchlorosilane: a visible light photocleavable alcohol protecting group. Chem. Sci. 2023, 14, 12615–12620.
- 31(a) Chen, Z.-H.; Su, X.-X.; Li, Q.; Wu, J.-Q.; Ou, T.-M.; Wang, H. Synthesis of α-Boryl Ketones via Hydration or Oxidation of B(MIDA)- Decorated Alkynes. Org. Lett. 2023, 25, 1099–1103; (b) Zheng, L.; Guo, X.; Li, Y.-C.; Wu, Y.; Xue, X.; Wang, P. Cu/SaBox-Catalyzed Photoinduced Coupling of Acylsilanes with Alkynes. Angew. Chem. Int. Ed. 2023, 62, e202216373.
- 32(a) Ito, K.; Tamashima, H.; Iwasawa, N.; Kusama, H. Photochemically promoted transition metal-free cross-coupling of acylsilanes with organoboronic esters. J. Am. Chem. Soc. 2011, 133, 3716–3719; (b) Ishida, K.; Yamazaki, H.; Hagiwara, C.; Abe, M.; Kusama, H. Efficient generation and synthetic applications of alkylsubstituted siloxycarbenes: suppression of norrish-type fragmentations of alkanoylsilanes by triplet energy transfer. Chem. Eur. J. 2020, 26, 1249–1253.
- 33(a) Zhou, G.; Guo, Z.; Liu, S.; Shen, X. Divergent Synthesis of Fluoroalkyl Ketones through Controlling the Reactivity of Organoboronate Complexes. J. Am. Chem. Soc. 2024, 146, 4026–4035; (b) Guo, Y.; Zhou, G. Synthesis of organofluorine compounds with acylsilanes. Chin. J. Chem. 2024, 42, 887–902.
- 34 Ye, J.-H.; Quach, L.; Paulisch, T.; Glorius, F. Visible-light-induced, metal-free carbene insertion into b−h bonds between acylsilanes and pinacolborane. J. Am. Chem. Soc. 2019, 141, 16227–16231.
- 35 Ueda, Y.; Masuda, Y.; Iwai, T.; Imaeda, K.; Takeuchi, H.; Ueno, K.; Gao, M.; Hasegawa, J.-Y.; Swamura, M. Photoinduced copper-catalyzed asymmetric acylation of allylic phosphates with acylsilanes. J. Am. Chem. Soc. 2022, 144, 2218–2224.
- 36 Zhang, Y.; Zhang, Y.; Ye, C.; Qi, X.; Wu, L.-Z.; Shen, X. Cascade cyclization of alkene-tethered acylsilanes and allylic sulfones enabled by unproductive energy transfer photocatalysis. Nat. Commun. 2022, 13, 6111–6121.
- 37 Lu, X.; Zhao, Q.; Zhang, H.; Xu, P.; Chen, X.; Liu, Z. Photo-induced catalyst-free formal carbon insertion of acylsilanes into B–B and B–Si bonds. Org. Chem. Front. 2024, 11, 2339–2343.




