Chiral Isothiourea-Catalyzed Acylative Dynamic Kinetic Resolution of 3-Hydroxyphthalides for Enantioselective Synthesis of Phthalidyl Esters
Zeyang Hao
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorWei Lin
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorZi-Qi Yuan
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorCorresponding Author
Wei Zhang
West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xin Li
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZeyang Hao
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorWei Lin
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorZi-Qi Yuan
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
Search for more papers by this authorCorresponding Author
Wei Zhang
West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xin Li
State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300192 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
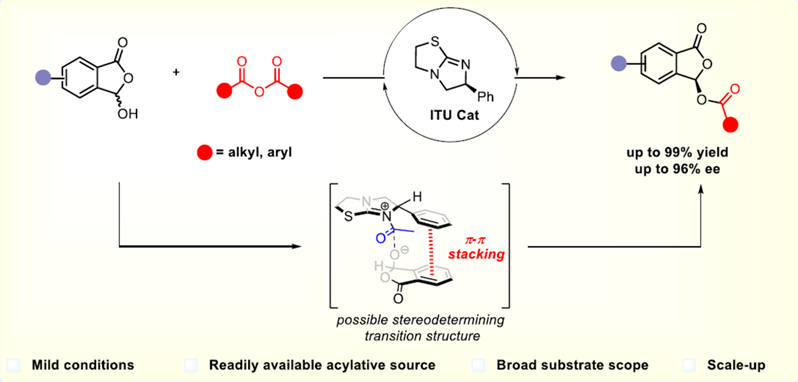
Phthalides serve as core structures pervasive in a wide array of natural products and drug molecules, which display a diverse array of biological activities. We report herein a highly efficient dynamic kinetic resolution of 3-hydroxyphthalides by chiral isothioureas (ITUs) catalyzed asymmetric acylation, facilitating the effective synthesis of a variety of chiral phthalidyl esters with good yields and enantioselectivities. Notably, this reaction features mild reaction conditions, expansive substrate scope as well as good functional group compatibility. In addition, the practicality of this method is underscored by the large-scale synthesis, reduced catalyst loading experiment and the synthesis of the chiral phthalidyl ester prodrug.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400382-sup-0001-supinfo.pdfPDF document, 11.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Majee, S.; Shilpa; Sarav, M.; Banik, B. K.; Ray, D. Recent Advances in the Green Synthesis of Active N-Heterocycles and Their Biological Activities. Pharmaceuticals 2023, 16, 873; (b) Zhang, J.-Y.; Chen, J.-Y.; Gao, C.-H.; Yu, L.; Ni, S.-F.; Tan, W.; Shi, F. Asymmetric (4+n) Cycloadditions of Indolyldimethanols for the Synthesis of Enantioenriched Indole-Fused Rings. Angew. Chem. Int. Ed. 2023, 62, e202305450; (c) Tan, J.-P.; Li, K.; Shen, B.; Zhuang, C.; Liu, Z.; Xiao, K.; Yu, P.; Yi, B.; Ren, X.; Wang, T. Asymmetric synthesis of Nbridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 2022, 13, 357; (d) Chen, Y.; He, J.; Zhuang, C.; Liu, Z.; Xiao, K.; Su, Z.; Ren, X.; Wang, T. Synergistic Catalysis between a Dipeptide Phosphonium Salt and a Metal-Based Lewis Acid for Asymmetric Synthesis of N-Bridged [3.2.1] Ring Systems Angew. Chem. Int. Ed. 2022, 61, e202207334; (e) Zhang, W.; Xiang, X.-X.; Chen, J.; Yang, C.; Pan, Y.-L.; Cheng, J.-P.; Meng, Q.; Li, X. Direct C-H Difluoromethylation of Heterocycles via Organic Photoredox Catalysis. Nat. Commun. 2020, 11, 638.
- 2 Brooks, W. H.; Guida, W. C.; Daniel, K. G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770.
- 3 Kumari Rayala, V. P.; Kandula, J. S.; Radhakrishnanand, P. Advances and challenges in the pharmacokinetics and bioanalysis of chiral drugs. Chirality 2022, 34, 1298–1310.
- 4(a) Maag, H. Prodrugs of Carboxylic Acids, Springer, New York, 2007;
10.1007/978-0-387-49785-3_20 Google Scholar(b) Bharate, S. S. Carboxylic Acid Counterions in FDA-Approved Pharmaceutical Salts. Pharm. Res. 2021, 38, 1307–1326; (c) Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933.
- 5(a) Sun, G.-Q.; Yu, P.; Zhang, W.; Zhang, W.; Wang, Y.; Liao, L. L.; Zhang, Z.; Li, L.; Lu, Z.; Yu, D.-G.; Lin, S. Electrochemical Reactor Dictates Site Selectivity in N−heteroarene Carboxylations. Nature 2023, 615, 67–72; (b) Zhang, W.; Chen, Z.; Jiang, Y.-X.; Liao, L.-L.; Wang, W.; Ye, J.-H.; Yu, D.-G. Nat. Commun. 2023, 14, 3529; (c) Cao, G.-M.; Hu, X.-L.; Liao, L.-L.; Yan, S.-S.; Song, L.; Chruma, J. J.; Gong, L.; Yu, D.-G. Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2. Nat. Commun. 2021, 12, 3306; (d) Chen, X.-W.; Yue, J.-P.; Wang, K.; Gui, Y.-Y.; Niu, Y.-N.; Liu, J.; Ran, C.-K.; Kong, W.; Zhou, W.-J.; Yu, D.-G. Nickel-Catalyzed Asymmetric Reductive Carbo-Carboxylation of Alkenes with CO2. Angew. Chem. Int. Ed. 2021, 60, 14068–14075; (e) Liao, L.-L.; Cao, G.-M.; Jiang, Y.-X.; Jin, X.-H.; Hu, X.-L.; Chruma, J. J.; Sun, G.-Q.; Gui, Y.-Y.; Yu, D.-G. α-Amino Acids and Peptides as Bifunctional Reagents: Carbocarboxylation of Activated Alkenes via Recycling CO2. J. Am. Chem. Soc. 2021, 143, 2812–2821.
- 6(a) Diao, X.; Pang, X.; Xie, C.; Guo, Z.; Zhong, D.; Chen, X. Bioactivation of 3-n-Butylphthalide via Sulfation of Its Major Metabolite 3-Hydroxy-NBP: Mediated Mainly by Sulfotransferase 1A1. Drug Metab. Dispos. 2014, 42, 774–781; (b) Dang, Q.; Brown, B. S.; van Poelje, P. D.; Colby, T. J.; Erion, M. D. Synthesis of Phosphonate 3-Phthalidyl Esters as Prodrugs for Potential Intracellular Delivery of Phosphonates. Bioorg. Med. Chem. Lett. 1999, 9, 1505–1510.
- 7 Luo, Q.; Tian, Z.; Tang, J.; Wang, J.; Tian, Y.; Peng, C.; Zhan, G.; Han, B. Design and Application of Chiral Bifunctional 4-Pyrrolidinopyridines: Powerful Catalysts for Asymmetric Cycloaddition of Allylic N-Ylide. ACS Catal. 2022, 12, 7221–7232.
- 8 Yamada, S.; Yamashita, K. Dynamic kinetic resolution of hemiaminals using a novel DMAP catalyst. Tetrahedron Lett. 2008, 49, 32–35.
- 9 Liu, Y.; Chen, Q.; Mou, C.; Pan, L.; Duan, X.; Chen, X.; Chen, H.; Zhao, Y.; Lu, Y.; Jin, Z.; Chi, Y. R. Catalytic asymmetric acetalization of carboxylic acids for access to chiral phthalidyl ester prodrugs. Nat. Commun. 2019, 10, 1675.
- 10 Liu, Y.; Majhi, P. K.; Song, R.; Mou, C.; Hao, L.; Chai, H.; Jin, Z.; Chi, Y. R. Carbene-Catalyzed Dynamic Kinetic Resolution and Asymmetric Acylation of Hydroxyphthalides and Related Natural Products. Angew. Chem. Int Ed. 2020, 59, 3859–3863.
- 11 Zhou, M.; Gridneva, T.; Zhang, Z.; He, E.; Liu, Y.; Zhang, W. Chiral Bicyclic Imidazole-Catalyzed Acylative Dynamic Kinetic Resolution for the Synthesis of Chiral Phthalidyl Esters. Angew Chem. Int Ed. 2021, 60, 1641–1645.
- 12When we were close to finish our study, Xie and Guo's group disclosed a closely related work with ArPNO as the organocatalyst. Chen, Y.-G.; Yu, H.-B.; Tian, Y.; Peng C.; Xie, M.-S.; Guo, H.-M. ArPNO-Catalyzed Acylative Dynamic Kinetic Resolution of 3-Hydroxyphthalides: Access to Enantioenriched Phthalidyl Esters. Org. Lett. 2023, 25, 5585–5590.
- 13 Merad, J.; Pons, J.-M.; Chuzel, O.; Bressy, C. Enantioselective Catalysis by Chiral Isothioureas. Eur. J. Org. Chem. 2016, 2016, 5589–5610.
- 14 Hassner, A.; Krepski, L. R.; Alexanian, V. Aminopyridines as Acylation Catalysts for Tertiary Alcohols. Tetrahedron 1978, 34, 2069–2076.
- 15 Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M. Z.; Smith, A. D. Organocatalytic Functionalization of Carboxylic Acids: Isothiourea-Catalyzed Asymmetric Intra- and Intermolecular Michael Addition−Lactonizations. J. Am. Chem. Soc. 2011, 133, 2714–2720.
- 16 Xu, L.-W.; Chen, Y.; Lu, Y. Catalytic Silylations of Alcohols: Turning Simple Protecting-Group Strategies into Powerful Enantioselective Synthetic Methods. Angew. Chem. Int. Ed. 2015, 54, 9456–9466.




