Photoinduced Perfluoroalkyloximation of Alkenes with Simple Perfluoroalkyl Halides
Wei Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorZhongji Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorDeliang Zhong
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorNianxing Wang
School of Chemistry and Pharmacy, Qilu University of Technology, Jinan, Shandong, 250353 China
Search for more papers by this authorCorresponding Author
Huaifeng Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
E-mail: [email protected]Search for more papers by this authorWei Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorZhongji Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorDeliang Zhong
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
These authors contributed equally to this work.
Search for more papers by this authorNianxing Wang
School of Chemistry and Pharmacy, Qilu University of Technology, Jinan, Shandong, 250353 China
Search for more papers by this authorCorresponding Author
Huaifeng Li
State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin, Guangxi, 541004 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
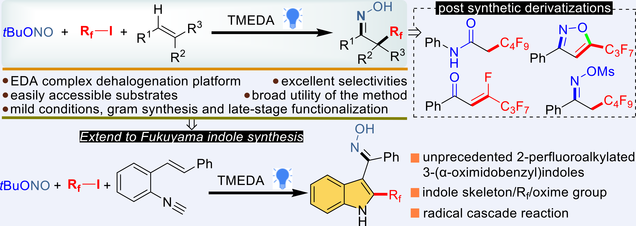
In this paper, the difunctionalizative perfluoroalkyloximation of alkenes has been developed for the first time. This photochemical method allows for the synthesis of various perfluoroalkyl ethanone oximes with excellent regioselectivity and good functional group tolerance. Our method employs the most common perfluoroalkyl source, perfluoroalkyl iodides, as Rf reagents. Besides long-chain perfluoroalkyl groups, this approach could be extended to incorporating additional groups, including trifluoromethyl, difluoromethyl, sulfonyl, and malonate, selectively into olefins, resulting in a range of β-substituted ethanone oximes. Notably, the potential of this method in the Fukuyama indole synthesis, generating novel 2-perfluoroalkylated 3-(α-oximidobenzyl)indoles via a radical cascade mechanism with 2-vinylphenylacryloyl isocyanate as the radical acceptor, presents a compelling avenue for drug synthesis. The protocol is efficient, scalable, and useful for late-stage modification of bioactive molecules.
Supporting Information
| Filename | Description |
|---|---|
| CJOC202400181-sup-0001-supinfo.pdfPDF document, 7.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Katakawa, K.; Kitajima, M.; Aimi, N.; Seki, H.; Yamaguchi, K.; Furihata, K.; Harayama, T.; Takayama, H. Structure elucidation and synthesis of lycoposerra-mine-B, a novel oxime-containing Lycopodium alkaloid from Lycopodium serratum Thunb. J. Org. Chem. 2005, 70, 658–663; (b) Calcul, L.; Inman, W. D.; Morris, A. A.; Tenney, K.; Ratnam, J.; McKerrow, J. H.; Valeriote, F. A.; Crews, P. Additional insights on the Bastadins: Isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configurations. J. Nat. Prod. 2010, 73, 365–372; (c) Walton, I.; Davis, M.; Munro, L.; Catalano, V. J.; Cragg, P. J.; Huggins, M. T.; Wallace, K. J. A Fluorescent Dipyrrinone Oxime for the Detection of Pesticides and Other Organophosphates. Org. Lett. 2012, 14, 2686–2689; (d) Cardoso-Martinez, F.; de la Rosa, J. M.; Diaz-Marrero, A. R.; Darias, J.; D'Croz, L.; Cerella, C.; Diederich, M.; Cueto, M. Oximoaspergillimide, a fungal derivative from a marine isolate of Aspergillus sp. Eur. J. Org. Chem. 2015, 2015, 2256–2261; (e) Qu, H.-E.; Huang, R.-Z.; Yao, G.-Y.; Li, J.-L.; Ye, M.-Y.; Wang, H.-S.; Liu, L. Synthesis and pharmacological evaluation of novel bisindole deriva-tives bearing oximes moiety: Identification of novel proapoptotic agents. Eur. J. Med. Chem. 2015, 95, 400–415; (f) Dong, J.; Hou, J.; Jiang, J.; Ai, S. Innovative approach for the electrochemical detection of non-electroactive organophosphorus pesticides using oxime as electroactive probe. Anal. Chim. Acta 2015, 885, 92–97; (g) Liu, N.; Song, L.; Liu, M.; Shang, F.; Anderson, Z.; Fox, D. J.; Challis, G. L.; Huang, Y. Unique post-translational oxime formation in the bio-synthesis of the azolemycin complex of novel ribosomal peptides from Streptomyces sp. FXJ1.264. Chem. Sci. 2016, 7, 482–488; (h) Lin, G.; Bai, X.; Duan, W.; Cen, B.; Huang, M.; Lu, S. High value-added application of sustainable natural forest product α-Pinene: Synthesis of myrtenal oxime esters as potential KARI inhibitors. ACS Sustainable Chem. Eng. 2019, 7, 7862–7868; (i) Motaleb, M. A.; Selim, A. A. Dioximes: Synthesis and biomedical applications. Bioorg. Chem. 2019, 82, 145–155.
- 2(a) Yang, S. H.; Chang, S. Highly efficient and catalytic conversion of aldoximes to nitriles. Org. Lett. 2001, 3, 4209–4211; (b) Owston, N. A.; Parker, A. J.; Williams, J. M. J. Highly efficient ruthenium-catalyzed oxime to amide rearrangement. Org. Lett. 2007, 9, 3599–3601; (c) Ramon, R. S.; Bosson, J.; Diez-Gonzalez, S.; Marion, N.; Nolan, S. P. Au/Ag-Cocatalyzed aldoximes to amides rearrangement under solvent- and acid-free conditions. J. Org. Chem. 2010, 75, 1197–1202; (d) Sukhorukov, A. Y.; Ioffe, S. L. Chemistry of six-membered cyclic oxime ethers. Application in the synthesis of bioactive compounds. Chem. Rev. 2011, 111, 5004–5041; (e) Shi, Z.; Koester, D. C.; Boultada-kis-Arapinis, M.; Glorius, F. Rh(III)-catalyzed synthesis of multisubstituted isoquinoline and pyridine N-oxides from oximes and diazo compounds. J. Am. Chem. Soc. 2013, 135, 12204–12207; (f) Xu, F.; Wang, C.; Wang, H.; Li, X.; Wan, B. Eco-friendly synthesis of pyridines via rhodium-catalyzed cyclization of diynes with oximes. Green Chem. 2015, 17, 799–803; (g) Kong, W.; Guo, Q.; Xu, Z.; Wang, G.; Jiang, X.; Wang, R. Iodine(III)-mediated oxy-fluorination of alkenyl oximes: An easy path to monofluoromethyl-substituted isoxazolines. Org. Lett. 2015, 17, 3686–3689; (h) Zhu, Z.; Tang, X.; Li, J.; Li, X.; Wu, W.; Deng, G.; Jiang, H. Iron-catalyzed synthesis of 2H-imidazoles from oxime acetates and vinyl azides under redox-neutral conditions. Org. Lett. 2017, 19, 1370–1373; (i) Dahiya, A.; Sahoo, A. K.; Alam, T.; Patel, B. K. tert-Butyl nitrite (TBN), a multitasking reagent in organic synthesis. Chem. Asian J. 2019, 14, 4454–4492.
- 3(a) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, UK, 2006;
10.1002/9780470988589 Google Scholar(b) Mueller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886; (c) Hird, M. Fluorinated liquid crystals - properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095; (d) Ma, J.-A.; Cahard, D. Update 1 of: Asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev. 2008, 108, 1–43; (e) Kirk, K. L. Fluorination in medicinal chemistry: Methods, strategies, and recent developments. Org. Process Res. Dev. 2008, 12, 305–321; (f) O'Hagan, D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319; (g) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity , Applications, Wiley-VCH, Weinheim, 2013;10.1002/9783527651351 Google Scholar(h) Reddy, V. P. Organofluorine Chemistry: Synthesis and Applications, Elsevier, 2020.
- 4(a) Surhone, L. M.; Tennoe, M. T.; Henssonow, S. F. Vicinal Difunctionalization, Betascript Publishing, 2010; (b) Yin, G.; Mu, X.; Liu, G. Palladium(II)-catalyzed oxidative difunctionalization of alkenes: Bond forming at a high-valent palladium center. Acc. Chem. Res. 2016, 49, 2413–2423; (c) Giri, R.; Kc, S. Strategies toward dicarbofunctionalization of unactivated olefins by combined Heck carbometalation and cross-coupling. J. Org. Chem. 2018, 83, 3013–3022; (d) Dhungana, R. K.; Kc, S.; Basnet, P.; Giri, R. Transition metal-catalyzed dicarbofunctionalization of unactivated olefins. Chem. Rec. 2018, 18, 1314–1340; (e) Mei, H.; Yin, Z.; Liu, J.; Sun, H.; Han, J. Recent advances on the electrochemical difunctionalization of alkenes/alkynes. Chin. J. Chem. 2019, 37, 292–301; (f) Wu, Y.-C.; Xiao, Y.-T.; Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Recent advances in silver-mediated radical difunctionalization of alkenes. ChemCatChem 2020, 12, 5312–5329; (g) Yu, X.-Y.; Zhao, Q.-Q.; Chen, J.; Xiao, W.-J.; Chen, J.-R. When light meets nitrogen-centered radicals: From reagents to catalysts. Acc. Chem. Res. 2020, 53, 1066–1083; (h) Li, Y.; Wu, D.; Cheng, H.-G.; Yin, G. Difunctionalization of alkenes involving metal migration. Angew. Chem. Int. Ed. 2020, 59, 7990–8003.
- 5(a) Guo, Q.; Wang, M.; Peng, Q.; Huo, Y.; Liu, Q.; Wang, R.; Xu, Z. Dual-functional chiral Cu-catalyst-induced photoredox asymmetric cyanofluoroalkylation of alkenes. ACS Catal. 2019, 9, 4470–4476; (b) Tang, L.; Yang, F.; Yang, Z.; Chen, H.; Cheng, H.; Zhang, S.; Zhou, Q.; Rao, W. Application of bifunctional 2-amino-1,4-naphthoquinones in visible-Light-promoted photocatalyst-free alkene perfluoroalkyl-alkenylation. Org. Lett. 2021, 23, 519–524; (c) Yang, H.-B.; Wang, Z.-H.; Li, J.-M.; Wu, C. Modular synthesis of α-aryl β-perfluoroalkyl ketones via N-heterocyclic carbene catalysis. Chem. Commun. 2020, 56, 3801–3804; (d) Zheng, D.; Studer, A. Photoinitiated three-component α-perfluoroalkyl-β-heteroarylation of unactivated alkenes via electron catalysis. Org. Lett. 2019, 21, 325–329; (e) Geng, X.; Lin, F.; Wang, X.; Jiao, N. Azidofluoroalkylation of alkenes with simple fluoroalkyl iodides enabled by photoredox catalysis. Org. Lett. 2017, 19, 4738–4741; (f) Chen, Y.; Li, L.; Ma, Y.; Li, Z. Cobalt-catalyzed three-component difluoroalkylation–peroxidation of alkenes. J. Org. Chem. 2019, 84, 5328–5338; (g) Zhang, P.; Li, W.; Qu, W.; Shu, Z.; Tao, Y.; Lin, J.; Gao, X. Copper and photocatalytic radical relay enabling fluoroalkylphosphorothiolation of alkenes: Modular synthesis of fluorine-containing S-alkyl phosphorothioates and phosphorodithioates. Org. Lett. 2021, 23, 9267–9272; (h) Zhang, B.-S.; Gao, L.-Y.; Zhang, Z.; Wen, Y.-H.; Liang, Y.-M. Three-component difluoroalkylation and trifluoromethylthiolation/trifluoromethylselenolation of π-bonds. Chem. Commun. 2018, 54, 1185–1188; (i) Rawner, T.; Lutsker, E.; Kaiser, C. A.; Reiser, O. The different faces of photoredox catalysts: Visible-light-mediated atom transfer radical addition (ATRA) reactions of perfluoroalkyl iodides with styrenes and phenylacetylenes. ACS Catal. 2018, 8, 3950–3956.
- 6(a) Lu, K.; Wei, X.; Li, Q.; Li, Y.; Ji, L.; Hua, E.; Dai, Y.; Zhao, X. Synthesis of α-trifluoromethyl ethanone oximes via the three-component reaction of aryl-substituted ethylenes, tert-butyl nitrite, and the Langlois reagent. Org. Chem. Front. 2019, 6, 3766–3770; (b) Wu, Y.; Zhang, Y.; Yang, Z.; Jiao, J.; Zheng, X.; Feng, W.; Zhang, M.; Cheng, H.; Tang, L. J. C. Dual roles of tert-butyl nitrite in the transition metal-and external oxidant-free trifluoromethyloximation of alkenes. ChemSusChem 2019, 12, 3960–3966.
- 7(a) Postigo, A. Electron donor-acceptor complexes in perfluoroalkylation reactions. Eur. J. Org. Chem. 2018, 2018, 6391–6404; (b) Yamada, S.; Konno, T. Recent advances in halogen bond-assisted organic synthesis. Curr. Org. Chem. 2020, 24, 2118–2152; (c) Yakubov, S.; Barham, J. P. Photosensitized direct C-H fluorination and trifluoromethylation in organic synthesis. Beilstein J. Org. Chem. 2020, 16, 2151–2192.
- 8 Baldwin, J. E. Thermal rearrangements of vinylcyclopropanes to cyclopentenes. Chem. Rev. 2003, 103, 1197–1212.
- 9(a) Tang, X.; Studer, A. Alkene 1,2-difunctionalization by radical alkenyl migration. Angew. Chem. Int. Ed. 2018, 57, 814–817;
(b) Liu, Y.; Chen, X.-L.; Sun, K.; Li, X.-Y.; Zeng, F.-L.; Liu, X.-C.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Visible-light induced radical perfluoroalkylation/cyclization strategy to access 2-perfluoroalkylbenzothiazoles/benzoselenazoles by EDA complex. Org. Lett. 2019, 21, 4019–4024;
(c) Chen, Z.-Y.; Lin, B.-Z.; Chen, L.; Zou, Y.; Yan, M.; Zhang, X.-J. Perfluorobutyl iodide mediated [1,2] and [2,3] Stevens rearrangement for the synthesis of indolin-3-ones. Adv. Synth. Catal. 2020, 362, 4368–4372;
(d) Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476;
(e) Varga, B.; Tóth, B. L.; Béke, F.; Csenki, J. T.; Kotschy, A.; Novák, Z. Synthesis and photochemical application of hydrofluoroolefin (HFO) based fluoroalkyl building block. Org. Lett. 2021, 23, 4925–4929;
(f) Tang, L.; Lv, G.; Fu, Y.; Chang, X.-P.; Cheng, R.; Wang, L.; Zhou, Q. Bifunctional 1,8-diazabicyclo[5.4.0]undec-7-ene for visible light-induced Heck-type perfluoroalkylation of alkenes. J. Org. Chem. 2022, 87, 14763–14777;
(g) Wang, Y.; Liu, R.; Zhou, P.; Wu, J.; Li, W.; Wang, C.; Li, H.; Li, D.; Yang, J. Visible-light- driven base-promoted radical cascade difluoroalkylation-cyclization- iodination of 1,6-enynes with ethyl difluoroiodoacetate. Eur. J. Org. Chem. 2022, 5, e202101395.
10.1002/ejoc.202101395 Google Scholar
- 10The tBuO radical could react with TMEDA radical cations or iodide ions (either through HAT from radical cations or reduction by I-) to produce tBuOH. Further efforts to understand the detailed mechanism of this method are ongoing.




