Heterometallic Mg-Ni-Mg Complex Promoted Hydrosilylation of Alkenes: Catalytic Performance and Intermediates Characterization†
Yanping Cai
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorShengjie Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xin Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]Search for more papers by this authorYanping Cai
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorShengjie Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xin Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
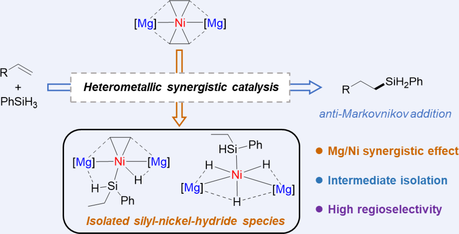
Heteronuclear metal complexes have played an increasingly important role in both small molecules activation and catalytic transformation due to the potential metal-metal synergies. In this work, we reported that the well-defined Mg-Ni-Mg complex [(LMg)2Ni(C2H4)2] {L = [(DippNCMe)2CH]−, Dipp = 2,6-iPr2C6H3} was capable of catalyzing the conversion of a diverse array of terminal alkenes to hydrosilylated products in anti-Markovnikov fashion using PhSiH3 as the silicon source. The stoichiometric reaction of heterometallic Mg-Ni-Mg complex with one equivalent PhSiH3 obtained a silyl-nickel-monohydride complex [(LMg)2NiH(C2H4)(SiHPhEt)] featuring a Ni-Si-H-Mg interaction. Moreover, treatment of heterometallic Mg-Ni-Mg complex with three equivalents PhSiH3 provided the silyl-nickel-trihydride complex [(LMg)2NiH3(SiHPhEt)] with three hydride-bridged at Mg-Ni-Mg fragment. Further reactions of the resultant silyl-nickel complexes with alkenes, e.g., ethylene and styrene, yielded the corresponding alkene-coordinated Mg-Ni-Mg complexes [(LMg)2Ni(C2H4)2], [(LMg)2NiH2(C2H4)] and [(LMg)2NiH2(CH2CHPh)], respectively, with the elimination of PhEtSiH2. Based on the control experiments, both silyl-nickel-monohydride and silyl-nickel-trihydride complexes were considered as active intermediates in the catalytic hydrosilylation reaction.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400176_sm_suppl.pdfPDF document, 3.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Marciniec, B. G.; Gulinski, J.; Urbaniak, W.; Kornetka, Z. W. In Comprehensive Handbook on Hydrosilylation, Ed.: Marciniec, B. G., Pergamon, Oxford, U.K., 1992.
- 2 Roy, A. K. In Advances in Organometallic Chemistry, Eds.: Robert West, A. F. H.; Mark, J. F., Academic Press, New York, 2007, Vol. 55, pp. 1–59.
- 3 Clarson, S. J. Bogdan Marciniec, Hydrosilylation: A Comprehensive Review on Recent Advances. Silicon 2009, 1, 57–58.
- 4 Zhu, S.-F. Catalytic Hydrogen Transfer Reactions. Chin. J. Chem. 2021, 39, 3211–3218.
- 5 Troegel, D.; Stohrer, J. Recent Advances and Actual Challenges in Late Transition Metal Catalyzed Hydrosilylation of Olefins from an Industrial Point of View. Coord. Chem. Rev. 2011, 255, 1440–1459.
- 6 Nakajima, Y.; Shimada, S. Hydrosilylation Reaction of Olefins: Recent Advances and Perspectives. RSC Adv. 2015, 5, 20603–20616.
- 7 Du, X.; Huang, Z. Advances in Base-Metal-Catalyzed Alkene Hydrosilylation. ACS Catal. 2017, 7, 1227–1243.
- 8 de Almeida, L. D.; Wang, H.; Junge, K.; Cui, X.; Beller, M. Recent Advances in Catalytic Hydrosilylations: Developments beyond Traditional Platinum Catalysts. Angew. Chem. Int. Ed. 2021, 60, 550–565.
- 9 Chen, J.; Guo, J.; Lu, Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018, 36, 1075–1109.
- 10 Sun, J.; Deng, L. Cobalt Complex-Catalyzed Hydrosilylation of Alkenes and Alkynes. ACS Catal. 2016, 6, 290–300.
- 11 Liu, D.; Liu, B.; Pan, Z.; Li, J.; Cui, C. Rare-earth Metal Catalysts for Alkene Hydrosilylation. Sci. China Chem. 2019, 62, 571–582.
- 12 Li, L.-J.; He, Y.; Yang, Y.; Guo, J.; Lu, Z.; Wang, C.; Zhu, S.; Zhu, S.-F. Recent Advances in Mn, Fe, Co, and Ni Catalyzed Organic Reactions. CCS Chem. 2024, 6, 537–584.
- 13 Huang, W.; Xu, L. Highly Construction of Tetrasubstituted Silylallenes via 1,4-Hydrosilylation of 1,3-Enynes. Chin. J. Org. Chem. 2021, 41, 4515–4516.
- 14 Chen, J.; Wu, J. Nickel-Catalyzed Enantioselective Hydrosilylation of gem-Difluoroalkenes with Silanes. Chin. J. Org. Chem. 2022, 42, 921–922.
- 15 Chalk, A. J.; Harrod, J. F. The Mechanism of the Hydrosilation of Olefins Catalyzed by Group VIII Metal Complexes. J. Am. Chem. Soc. 1965, 87, 16–21.
- 16 Schroeder, M. A.; Wrighton, M. S. Pentacarbonyliron(0) Photocatalyzed Reactions of Trialkylsilanes with Alkenes. J. Organomet. Chem. 1977, 128, 345–358.
- 17 Xie, S.; Dong, Y.; Du, X.; Fan, Q.; Yang, H.; Li, X.; Sun, H.; Fuhr, O.; Fenske, D. Solvent-Free Hydrosilylation of Alkenes Catalyzed by Well-Defined Low-Valent Cobalt Catalysts. Organometallics 2021, 40, 286–293.
- 18 Bories, C. C.; Barbazanges, M.; Derat, E.; Petit, M. Implication of a Silyl Cobalt Dihydride Complex as a Useful Catalyst for the Hydrosilylation of Imines. ACS Catal. 2021, 11, 14262–14273.
- 19 Li, Y.; Krause, J. A.; Guan, H. Silane Activation with Cobalt on the PoCOP Pincer Ligand Platform. Organometallics 2020, 39, 3721–3730.
- 20 Metsänen, T. T.; Gallego, D.; Szilvási, T.; Driess, M.; Oestreich, M. Peripheral Mechanism of a Carbonyl Hydrosilylation Catalysed by an SiNSi Iron Pincer Complex. Chem. Sci. 2015, 6, 7143–7149.
- 21 Scheuermann, M. L.; Semproni, S. P.; Pappas, I.; Chirik, P. J. Carbon Dioxide Hydrosilylation Promoted by Cobalt Pincer Complexes. Inorg. Chem. 2014, 53, 9463–9465.
- 22 MacMillan, S. N.; Harman, W. H.; Peters, J. C. Facile Si-H Bond Activation and Hydrosilylation Catalysis Mediated by a Nickel-Borane Complex. Chem. Sci. 2014, 5, 590–597.
- 23 Bart, S. C.; Lobkovsky, E.; Chirik, P. J. Preparation and Molecular and Electronic Structures of Iron(0) Dinitrogen and Silane Complexes and Their Application to Catalytic Hydrogenation and Hydrosilation. J. Am. Chem. Soc. 2004, 126, 13794–13807.
- 24 Amaya, A. L.; Alawisi, H.; Arman, H. D.; Tonzetich, Z. J. Well-Defined Cobalt-Silyl Complexes and Their Role in Catalytic Carbonyl Hydrosilylation. Organometallics 2023, 42, 2902–2909.
- 25 Fan, Q.; Du, X.; Yang, W.; Li, Q.; Huang, W.; Sun, H.; Hinz, A.; Li, X. Effects of Silylene Ligands on the Performance of Carbonyl Hydrosilylation Catalyzed by Cobalt Phosphine Complexes. Dalton Trans. 2023, 52, 6712–6721.
- 26 Sen, A.; Kumar, R.; Tewari, T.; Gonnade, R. G.; Chikkali, S. H. Iron-Catalyzed Alkoxylation, Dehydrogenative-Polymerization and Tandem Hydrosilylative-Alkoxylation. Chem. Eur. J. 2023, 29, e202301375.
- 27 Mankad, N. P. Diverse Bimetallic Mechanisms Emerging from Transition Metal Lewis Acid/Base Pairs: Development of co-Catalysis with Metal Carbenes and Metal Carbonyl Anions. Chem. Commun. 2018, 54, 1291–1302.
- 28 Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic Catalysis Based on Heterometallic Complexes and Clusters. Chem. Rev. 2015, 115, 28–126.
- 29 Pye, D. R.; Mankad, N. P. Bimetallic Catalysis for C-C and C-X Coupling Reactions. Chem. Sci. 2017, 8, 1705–1718.
- 30 Chatterjee, B.; Chang, W.-C.; Jena, S.; Werlé, C. Implementation of Cooperative Designs in Polarized Transition Metal Systems-Significance for Bond Activation and Catalysis. ACS Catal. 2020, 10, 14024–14055.
- 31 Li, N.; Zhang, W.-X. Frustrated Lewis Pairs: Discovery and Overviews in Catalysis. Chin. J. Chem. 2020, 38, 1360–1370.
- 32 Wang, Y.-X.; Wang, H.-M.; Meng, P.; Song, D.-X.; Qi, Z.; Zhang, X.-M. Fe2Mn(μ3-O)(COO)6 Cluster Based Stable MOF for Oxidative Coupling of Amines via Heterometallic Synergy. Chin. J. Chem. 2021, 39, 2983–2989.
- 33 Takaya, J. Catalysis Using Transition Metal Complexes Featuring Main Group Metal and Metalloid Compounds as Supporting Ligands. Chem. Sci. 2021, 12, 1964–1981.
- 34 Hara, N.; Saito, T.; Semba, K.; Kuriakose, N.; Zheng, H.; Sakaki, S.; Nakao, Y. Rhodium Complexes Bearing PAlP Pincer Ligands. J. Am. Chem. Soc. 2018, 140, 7070–7073.
- 35 Cai, Y.; Jiang, S.; Dong, L.; Xu, X. Synthesis and Reactivity of Heterometallic Complexes Containing Mg- or Zn-Metalloligands. Dalton Trans. 2022, 51, 3817–3827.
- 36 Shoshani, M. M. Cooperative Heterometallic Platforms Enabling Selective C-H Bond Activation and Functionalization of Pyridines. Cell Rep. Phys. Sci. 2023, 4, 101213.
- 37 Hara, N.; Semba, K.; Nakao, Y. X-Type Aluminyl Ligands for Transition-Metal Catalysis. ACS Catal. 2022, 12, 1626–1638.
- 38 Bouhadirab, G.; Bourissou, D. Complexes of Ambiphilic Ligands: Reactivity and Catalytic Applications. Chem. Soc. Rev. 2016, 45, 1065–1079.
- 39 Muhr, M.; Liang, H.; Allmendinger, L.; Bühler, R.; Napoli, F. E.; Ukaj, D.; Cokoja, M.; Jandl, C.; Kahlal, S.; Saillard, J.-Y.; Gemel, C.; Fischer, R. A. Catalytic Alkyne Semihydrogenation with Polyhydride Ni/Ga Clusters. Angew. Chem. Int. Ed. 2023, 62, e202308790.
- 40 De Leon, E.; Gonzalez, F.; Bauskar, P.; Gonzalez-Eymard, S.; De Los Santos, D.; Shoshani, M. M. Amplifying Reactivity of Metal Hydrides: A Heterotrimetallic NiAl2(μ2-H)2 Catalyst for the Dearomatization of N-Heterocycles. Organometallics 2023, 42, 435–440.
- 41 Cai, Y.; Jiang, S.; Rajeshkumar, T.; Maron, L.; Xu, X. A Planar Nickelaspiropentane Complex with Magnesium-Based Metalloligands: Synthesis, Structure, and Synergistic Dihydrogen Activation. J. Am. Chem. Soc. 2022, 144, 16647–16655.
- 42 Takaya, J.; Iwasawa, N. Synthesis, Structure, and Catalysis of Palladium Complexes Bearing a Group 13 Metalloligand: Remarkable Effect of an Aluminum-Metalloligand in Hydrosilylation of CO2. J. Am. Chem. Soc. 2017, 139, 6074–6077.
- 43 Bajo, S.; Theulier, C. A.; Campos, J. Mechanistic Investigations on Hydrogenation, Isomerization and Hydrosilylation Reactions Mediated by a Germyl-Rhodium System. ChemCatChem 2022, 14, e202200157.
- 44 Steinhoff, P.; Paul, M.; Schroers, J. P.; Tauchert, M. E. Highly Efficient Palladium-Catalysed Carbon Dioxide Hydrosilylation Employing PMP Ligands. Dalton Trans. 2019, 48, 1017–1022.
- 45 Jiang, S.; Chen, M.; Xu, X. Formation of Zn-Zn and Zn-Pd Bonded Complexes by Reactions of Terminal Zinc Hydrides with Pd(II) Species. Inorg. Chem. 2019, 58, 13213–13220.
- 46 Cordero, B.; Gómez, V.; Platero-Prats, A. E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent Radii Revisited. Dalton Trans. 2008, 2832–2838.
- 47 Huang, J.; Zheng, X.; Del Rosal, I.; Zhao, B.; Maron, L.; Xu, X. Nickel(0)-Induced β-H Elimination of Magnesium Alkyls: Formation and Reactivity of Heterometallic Hydrides. Inorg. Chem. 2020, 59, 13473–13480.
- 48 Garçon, M.; Bakewell, C.; Sackman, G. A.; White, A. J. P.; Cooper, R. I.; Edwards, A. J.; Crimmin, M. R. A Hexagonal Planar Transition-Metal Complex. Nature 2019, 574, 390–393.
- 49 Shoshani, M. M.; Liu, J.; Johnson, S. A. Mechanistic Insight into H/D Exchange by a Pentanuclear Ni-H Cluster and Synthesis and Characterization of Structural Analogues of Potential Intermediates. Organometallics 2018, 37, 116–126.
- 50 Harder, S.; Spielmann, J.; Intemann, J.; Bandmann, H. Hydrogen Storage in Magnesium Hydride: The Molecular Approach. Angew. Chem. Int. Ed. 2011, 50, 4156–4160.
- 51 Mukherjee, D.; Okuda, J. Molecular Magnesium Hydrides. Angew. Chem. Int. Ed. 2018, 57, 1458–1473.
- 52
Green, S. P.; Jones, C.; Stasch, A. Stable Adducts of a Dimeric Magnesium(I) Compound. Angew. Chem. Int. Ed. 2008, 120, 9219–9223.
10.1002/ange.200803960 Google Scholar
- 53 Coates, G.; Tan, H. Y.; Kalff, C.; White, A. J. P.; Crimmin, M. R. Defluorosilylation of Industrially Relevant Fluoroolefins Using Nucleophilic Silicon Reagents. Angew. Chem. Int. Ed. 2019, 58, 12514–12518.
- 54 Goddard, R.; Krüger, C.; Ramadan, N. A.; Ritter, A. Silicon Analogues of Grignard Compounds: Synthesis and Structure of Amine-Stabilized Trimethylsilylmagnesium Halides. Angew. Chem. Int. Ed. 1995, 34, 1030–1032.
- 55 Liptrot, D. J.; Arrowsmith, M.; Colebatch, A. L.; Hadlington, T. J.; Hill, M. S.; Kociok-Köhn, G.; Mahon, M. F. Beyond Dehydrocoupling: Group 2 Mediated Boron-Nitrogen Desilacoupling. Angew. Chem. Int. Ed. 2015, 54, 15280–15283.
- 56 Okokhere-Edeghoghon, B.; Hill, M. S.; Mahon, M. F.; McMullin, C. L. Diverse Reactivity of a Magnesium Silanide toward Ketones. Chem. Commun. 2022, 58, 10711–10714.
- 57 Schnitzler, S.; Spaniol, T. P.; Maron, L.; Okuda, J. Formation and Reactivity of a Molecular Magnesium Hydride with a Terminal Mg-H Bond. Chem. Eur. J. 2015, 21, 11330–11334.
- 58 Eberhardt, N. A.; Guan, H. Nickel Hydride Complexes. Chem. Rev. 2016, 116, 8373–8426.
- 59 Garçon, M.; White, A. J. P.; Crimmin, M. R. Palladium-Catalysed Magnesiation of Benzene. Chem. Commun. 2018, 54, 12326–12328.
- 60 Gao, Y.; Wang, L.; Deng, L. Distinct Catalytic Performance of Cobalt(I)-N-Heterocyclic Carbene Complexes in Promoting the Reaction of Alkene with Diphenylsilane: Selective 2,1-Hydrosilylation, 1,2-Hydrosilylation, and Hydrogenation of Alkene. ACS Catal. 2018, 8, 9637–9646.




