Unique Azolyl Acylhydrazonyl Hybridization of Aloe Emodins to Access Potential Antibacterial Agents
Yi-Xin Wang
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
These authors contributed equally to this work.
Search for more papers by this authorZhao Deng
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
These authors contributed equally to this work.
Search for more papers by this authorAisha Bibi
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
Search for more papers by this authorCorresponding Author
Bo Fang
College of Pharmacy, National & Local Joint Engineering Research Center of Targeted and Innovative Therapeutics, Chongqing Key Laboratory of Kinase Modulators as Innovative Medicine, Chongqing University of Arts and Sciences, Chongqing, 402160 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Cheng-He Zhou
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
E-mail: [email protected], [email protected]Search for more papers by this authorYi-Xin Wang
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
These authors contributed equally to this work.
Search for more papers by this authorZhao Deng
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
These authors contributed equally to this work.
Search for more papers by this authorAisha Bibi
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
Search for more papers by this authorCorresponding Author
Bo Fang
College of Pharmacy, National & Local Joint Engineering Research Center of Targeted and Innovative Therapeutics, Chongqing Key Laboratory of Kinase Modulators as Innovative Medicine, Chongqing University of Arts and Sciences, Chongqing, 402160 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Cheng-He Zhou
Institute of Bioorganic & Medicinal Chemistry, Key Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 China
E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
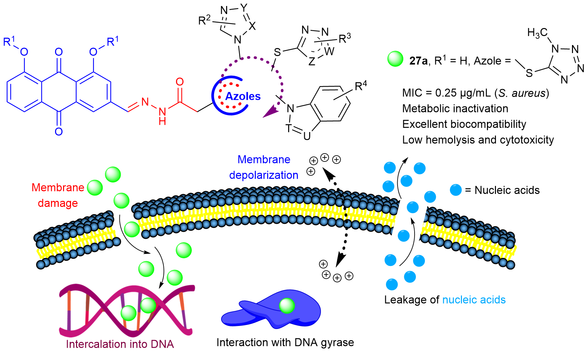
A type of unique azole-hybridized acylhydrazonyl aloe emodins (AAEs) were developed as new antibacterial agents for combating bacterial infections. Some target AAEs showed strong antibacterial activities, especially, tetrazolylthioether AAE 27a exhibited broad antibacterial spectrum with 16—256 folds and 8—64 folds more active antibacterial efficacy than the reference drugs aloe emodin and norfloxacin, respectively. Tetrazolylthioether AAE 27a also gave low hemolysis and cytotoxicity, as well as favorable bioavailability. Preliminary mechanism explorations revealed that tetrazolylthioether AAE 27a could cause bacterial membrane depolarization and damage the cell membrane, resulting in nucleic acid leakage. Moreover, compound 27a could intercalate into DNA to impede its replication and form supramolecular 27a-DNA gyrase complex to disturb the function of DNA gyrase. These findings would provide valuable insights for the further exploration of azolyl acylhydrazonyl aloe emodins as new potential antibacterial candidates.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400160-sup-0001-supinfo.pdfPDF document, 4.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Porras, G.; Chassagne, F.; Lyles, J. T.; Marquez, L.; Dettweiler, M.; Salam, A. M.; Samarakoon, T.; Shabih, S.; Farrokhi, D. R.; Quave, C. L. Ethnobotany and The Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2020, 121, 3495−3560; (b) Salehi, B.; Albayrak, S.; Antolak, H.; Kregiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Fokou, P. V. T.; Yousef, Z.; Zakaria, Z. A.; Varoni, E. M.; Sharopov, F.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843; (c) Zargar, B. A.; Masoodi, M. H.; Ahmed, B.; Ganie, S. A. Phytoconstituents and Therapeutic Uses of Rheum Emodi Wall. ex Meissn. Food Chem. 2011, 128, 585−589.
- 2(a) Karatoprak, G. Ş.; Akkol, E. K.; Yücel, Ç.; Acıkara, Ö. B.; Sobarzo-Sánchez, E. Advances in Understanding the Role of Aloe Emodin and Targeted Drug Delivery Systems in Cancer. Oxid. Med. Cell. Longevity 2022, 2022, 7928200; (b) Chen, R. E.; Zhang, J. M.; Hu, Y. Y.; Wang, S. P.; Chen, M. W.; Wang, Y. T. Potential Antineoplastic Effects of Aloe-Emodin: A Comprehensive Review. Am. J. Chin. Med. 2014, 42, 275−288; (c) Srinivas, G.; Babykutty, S.; Satbiadevan, P. P.; Srinivas, P. Molecular Mechanism of Emodin Action: Transition from Laxative Ingredient to an Antitumor Agent. Med. Res. Rev. 2007, 27, 591−608.
- 3(a) Jiang, L. X.; Yi, T.; Shen, Z. Y.; Teng, Z. H.; Wang, J. F. Aloe-Emodin Attenuates Staphylococcus aureus Pathogenicity by Interfering with The Oligomerization of Α-Toxin. Front. Cell. Infect. Microbiol. 2019, 9, 157−166; (b) Hu, Y.; Yang, L.; Lai, Y. Recent Findings Regarding the Synergistic Effects of Emodin and Its Analogs with Other Bioactive Compounds: Insights Into New Mechanisms. Biomed. Pharmacother. 2023, 162, 114585; (c) Españo, E.; Kim, J.; Kim, J. K. Utilization of Aloe Compounds in Combatting Viral Diseases. Pharmaceuticals 2022, 15, 599; (d) Liu, L.; Kong, Y. Q.; He, L.; Wang, X. X.; Wang, M. M.; Xu, H. J.; Yang, C. G.; Su, Z.; Zhao, J.; Mao, Z. W.; Huang, Y.; Liu, H. K. A Rhein-Based Rh(III) Arene Complex with Anti-tumor Cell Proliferative Activity Inhibits RNA Demethylase FTO. Chin. J. Chem. 2022, 40, 1156–1164.
- 4(a) Dong, X. X.; Zeng, Y. W.; Liu, Y.; You, L. T.; Yin, X. B.; Fu, J.; Ni, J. Aloe-Emodin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Phytother. Res. 2020, 34, 270–281; (b) Jiang, H. Y.; Tang, W. Y. U.; Song, Y.; Jin, W.; Du, Q. Y. Induction of Apoptosis by Metabolites of Rhei Radix Et Rhizoma (Da Huang): A Review of The Potential Mechanism in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 806175.
- 5(a) Deng, Z.; Bheemanaboina, R. R. Y.; Luo, Y.; Zhou, C. H. Aloe Emodin-Conjugated Sulfonyl Hydrazones as Novel Type of Antibacterial Modulators Against S. aureus 25923 Through Multifaceted Synergistic Effects. Bioorg. Chem. 2022, 127, 106035; (b) Deng, Z.; Sun, H.; Bheemanaboina, R. R. Y.; Luo, Y.; Zhou, C. H. Natural Aloe Emodin-Hybridized Sulfonamide Aminophosphates as Novel Potential Membrane-Perturbing and DNA-Intercalating Agents Against Enterococcus faecalis. Bioorg. Med. Chem. Lett. 2022, 64, 128695.
- 6 Liang, X. Y.; Battini, N.; Sui, Y. F.; Ansari, M. F.; Gan, L. L.; Zhou, C. H. Aloe-Emodin Derived Azoles as A New Structural Type of Potential Antibacterial Agents: Design, Synthesis, and Evaluation of the Action on Membrane, DNA, and MRSA DNA Isomerase. RSC Med. Chem. 2021, 12, 602−608.
- 7(a) Brum, J. D. C.; França, T. C. C.; Villar, J. D. F. Synthesis and Biological Activity of Hydrazones and Derivatives: A Review. Mini-Rev. Med. Chem. 2020, 20, 342–368; (b) Popiolek, L. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389; (c) Liu, X.; Hamon, J. R. Recent Developments in Penta-, Hexa- and Heptadentate Schiff Base Ligands and Their Metal Complexes. Coord. Chem. Rev. 2019, 389, 94–118.
- 8(a) Teneva, Y.; Simeonova, R.; Valcheva, V.; Angelova, V. T. Recent Advances in Anti-tuberculosis Drug Discovery Based on Hydrazide-Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals 2023, 16, 484; (b) Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Quinoline Hydrazide/Hydrazone Derivatives: Recent Insights on Antibacterial Activity and Mechanism of Action. ChemMedChem 2023, 18, 202200571; (c) Singh, H.; Kumar, R.; Yadav, R. K.; Mazumder, A.; Salahuddin; Chauhan, B.; Abdullah, M. M. Insight into the Synthesis, Biological Activity, and Structure-Activity Relationship of Benzothiazole and Benzothiazole-Hydrazone Derivatives: A Comprehensive Review. Mini-Rev. Med. Chem. 2023, 23, 537–575.
- 9(a) Li, Y.; Sui, J. F.; Cui, L. S.; Jiang, H. L. Hydrogen Bonding Regulated Flexibility and Disorder in Hydrazone-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 1359–1366; (b) Zhao, W. H.; Xu, J. H.; Tangadanchu, V. K. R.; Zhou, C. H. Thiazolyl Hydrazineylidenyl Indolones as Unique Potential Multitargeting Broad- Spectrum Antimicrobial Agents. Eur. J. Med. Chem. 2023, 256, 115452; (c) Li, F. F.; Zhao, W. H.; Tangadanchu, V. K. R.; Meng, J. P.; Zhou, C. H. Discovery of Novel Phenylhydrazone-Based Oxindole- Thiolazoles as Potent Antibacterial Agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521.
- 10(a) Omidi, S.; Kakanejadifard, A. A Review on Biological Activities of Schiff Base, Hydrazone, and Oxime Derivatives of Curcumin. RSC Adv. 2020, 10, 30186–30202; (b) Liang, J. C.; Fu, X.; Zhang, J.; Ding, H. X.; Xu, Z. Y.; Ye, H. C.; Zhu, F. D.; Yan, C.; Gan, X. H.; Feng, G. Design, Synthesis and Antibacterial Activity of Coumarin-3-carboxylic Acid Derivatives Containing Acylhydrazone Moiety. Arabian J. Chem. 2023, 17, 105389; (c) Varshney, K.; Gupta, A. K.; Sonkar, R.; Varshney, S.; Mishra, A.; Bhatia, G.; Gaikwad, A.; Srivastava, A. K.; Saxena, M.; Jain, S.; Saxena, A. K. Lipid Lowering Oxopropanylindole Hydrazone Derivatives with Anti-oxidant and Anti-hyperglycemic Activity. Curr. Top. Med. Chem. 2019, 18, 2256–2265.
- 11(a) Sonawane, S. J.; Kalhapure, R. S.; Govender, T. Hydrazone Linkages in pH Responsive Drug Delivery Systems. Eur. J. Pharm. Sci. 2017, 99, 45–65; (b) Kumar, S.; Oh, J. M.; Prabhakaran, P.; Awasti, A.; Kim, H.; Mathew, B. Isatin-Tethered Halogen-Containing Acylhydrazone Derivatives as Monoamine Oxidase Inhibitor with Neuroprotective Effect. Sci. Rep. 2024, 14, 1264; (c) Angelova, V.; Karabeliov, V.; Andreeva-Gateva, P. A.; Tchekalarova, J. Recent Developments of Hydrazide/Hydrazone Derivatives and Their Analogs as Anticonvulsant Agents in Animal Models. Drug Dev. Res. 2016, 77, 379–392; (d) Peng, X. M.; Damu, G. L. V.; Zhou, C. H. Current Developments of Coumarin Compounds in Medicinal Chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930.
- 12(a) Fan, Y. L.; Jin, X. H.; Huang, Z. P.; Yu, H. F.; Zeng, Z. G.; Gao, T.; Feng, L. S. Recent Advances of Imidazole-Containing Derivatives as Antitubercular Agents. Eur. J. Med. Chem. 2018, 150, 347–365; (b) Zhang, L.; Peng, X. M.; Damu, G. L.; Geng, R. X.; Zhou, C. H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340−437; (c) Dai, J.; Battini, N.; Zang, Z. L.; Luo, Y.; Zhou, C. H. Discovery of Alkaloid Quinazolone-Derived Imidazolenones with Novel Structural Scaffolds of Multitargeting Antibacterial Potential. Chin. J. Chem. 2023, 41, 3645–3661.
- 13(a) Tolomeu, H. V.; Fraga, C. A. M. Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838; (b) Yang, X.; Sun, H.; Maddili, S. K.; Li, S.; Yang, R. G.; Zhou, C. H. Dihydropyrimidinone Imidazoles as Unique Structural Antibacterial Agents for Drug-Resistant Gram-Negative Pathogens. Eur. J. Med. Chem. 2022, 232, 114188; (c) Li, S. R.; Tan, Y. M.; Zhang, L.; Zhou, C. H. Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes. Pharmaceutics 2023, 15, 1348.
- 14(a) Kouser, S.; Hezam, A.; Khadri, M. J. N.; Khanum, S. A. A Review on Zeolite Imidazole Frameworks: Synthesis, Properties, and Applications. J. Porous Mater. 2022, 29, 663–681; (b) Li, F. F.; Zhang, P. L.; Tangadanchu, V. K. R.; Li, S.; Zhou, C. H. Novel Metronidazole-Derived Three-Component Hybrids as Promising Broad-Spectrum Agents to Combat Oppressive Bacterial Resistance. Bioorg. Chem. 2022, 122, 105718; (c) Peng, X. M.; Cai, G. X.; Zhou, C. H. Recent Developments in Azole Compounds as Antibacterial and Antifungal Agents. Curr. Top. Med. Chem. 2013, 13, 1963−2010.
- 15(a) Emami, L.; Faghih, Z.; Ataollahi, E.; Sadeghian, S.; Rezaei, Z.; Khabnadideh, S. Azole derivatives: recent advances as potent antibacterial and antifungal agents. Curr. Med. Chem. 2023, 30, 220–249; (b) Cheng, Y.; Wang, H.; Addla, D.; Zhou, C. H. Current Researches and Applications of Azole-based Supermolecules as Medicinal Agents. Chin. J. Org. Chem. 2016, 36, 1–42; (c) Zhang, H. Z.; Gan, L. L.; Wang, H.; Zhou, C. H. New Progress in Azole Compounds as Antimicrobial Agents. Mini-Rev. Med. Chem. 2017, 17, 122–166.
- 16(a) Tian, G. M.; Song, Q. Y.; Liu, Z. W.; Guo, J.; Cao, S.; Long, S. H. Recent Advances in 1,2,3- and 1,2,4-Triazole Hybrids as Antimicrobials and Their SAR: A Critical Review. Eur. J. Med. Chem. 2023, 259, 115603; (b) Zhang, H. Z.; Damu, G. L. V.; Cai, G. X.; Zhou, C. H. Current Developments in the Syntheses of 1,2,4-Triazole Compounds. Curr. Org. Chem. 2014, 18, 359–406; (c) Zhou, C. H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280.
- 17(a) Neochoritis, C. G.; Zhao, T.; Domling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042; (b) Dai, L. L.; Cui, S. F.; Damu, G. L. V.; Zhou, C. H. Recent Advances in the Synthesis and Application of Tetrazoles. Chin. J. Org. Chem. 2013, 33, 224–244.
- 18(a) Zhang, L.; Kumar, K. V.; Rasheed, S.; Zhang, S. L.; Geng, R. X.; Zhou, C. H. Design, Synthesis, and Antibacterial Evaluation of Novel Azolylthioether Quinolones as MRSA DNA Intercalators. MedChemComm 2015, 6, 1303–1310; (b) Li, P.; Yang, Y.; Wang, X.; Wu, X. Z. Recent Achievements on the Agricultural Applications of Thioether Derivatives: A 2010-2020 Decade in Review. J. Heterocycl. Chem. 2021, 58, 1225–1251; (c) Xie, Y. P.; Sangaraiah, N.; Meng, J. P.; Zhou, C. H. Unique Carbazole-Oxadiazole Derivatives as New Potential Antibiotics for Combating Gram-Positive and -Negative Bacteria. J. Med. Chem. 2022, 65, 6171–6190.
- 19(a) Otero-Ramirez, M. E.; Passioura, T.; Suga, H. Structural Features and Binding Modes of Thioether-Cyclized Peptide Ligands. Biomedicines 2019, 6, 116; (b) Lohans, C. T.; Vederas, J. C. Structural Characterization of Thioether-Bridged Bacteriocins. J. Antibiot. 2014, 67, 23–30.
- 20(a) Yadav, G.; Ganguly, S. Structure Activity Relationship (SAR) Study of Benzimidazole Scaffold for Different Biological Activities: A Mini-Review. Eur. J. Med. Chem. 2015, 97, 419–443; (b) Sun, H.; Li, Z. Z.; Jeyakkumar, P.; Zang, Z. L.; Fang, B.; Zhou, C. H. A New Discovery of Unique 13-(Benzimidazolylmethyl)berberines as Promising Broad- Spectrum Antibacterial Agents. J. Agric. Food Chem. 2022, 70, 12320−12329; (c) Wang, J.; Zhang, P. L.; Ansari, M. F.; Li, S.; Zhou, C. H. Molecular Design and Preparation of 2-Aminothiazole Sulfanilamide Oximes as Membrane Active Antibacterial Agents for Drug Resistant Acinetobacter baumannii. Bioorg. Chem. 2021, 113, 105039; (d) Zhang, J.; Tan, Y. M.; Li, S. R.; Battini, N.; Zhang, S. L.; Lin, J. M.; Zhou, C. H. Discovery of Benzopyridone Cyanoacetates as New Type of Potential Broad-Spectrum Antibacterial Candidates. Eur. J. Med. Chem. 2024, 265, 116107.
- 21(a) Wang, L.; Chen, S. Y.; Gao, X.; Liang, X.; Lv, W. C.; Zhang, D. F.; Jin, X. Recent Progress in Chemistry and Bioactivity of Monoterpenoid Indole Alkaloids from The Genus Gelsemium: A Comprehensive Review. J. Enzyme Inhib. Med. Chem. 2023, 38, 2155639; (b) Zhou, X. M.; Li, Q. Y.; Lu, X.; Bheemanaboina, R. R. Y.; Fang, B.; Cai, G. X.; Zhou, C. H. Identification of Unique Indolylcyanoethylenyl Sulfonylanilines as Novel Structural Scaffolds of Potential Antibacterial Agents. Eur. J. Med. Chem. 2023, 260, 115773; (c) Sun, H.; Ansari, M. F.; Fang, B.; Zhou, C. H. Natural Berberine-Hybridized Benzimidazoles as Novel Unique Bactericides Against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 7831–7840; (d) Lu, X.; Liu, Y. J.; Qin, N.; Du, C. X.; Hu, Y. Y.; Chen, Y.; Sun, H. P. Discovery of Tryptophan-tetrahydroisoquinoline Derivatives as Multifunctional Agents for Treatment of Alzheimer's Disease. Chin. J. Chem. 2022, 40, 1821–1830.
- 22(a) Kassab, A. E. Benzotriazole Scaffold: An Overview of Antiproliferative Potential, Mechanisms of Action, and Structure-Activity Relationships. Arch. Pharm. 2023, 356, 2300102; (b) Loukopoulos, E.; Kostakis, G. E. Recent Advances in The Coordination Chemistry of Benzotriazole-Based Ligands. Coord. Chem. Rev. 2019, 395, 193–229; (c) Zhou, X. M.; Hu, Y. Y.; Fang, B.; Zhou, C. H. Benzenesulfonyl Thiazoloimines as Unique Multitargeting Antibacterial Agents towards Enterococcus faecalis. Eur. J. Med. Chem. 2023, 248, 115088.
- 23(a) Wang, J.; Ansari, M. F.; Zhou, C. H. Identification of Unique Quinazolone Thiazoles as Novel Structural Scaffolds for Potential Gram-negative Bacterial Conquerors. J. Med. Chem. 2021, 64, 7630–7645; (b) Sui, Y. F.; Ansari, M. F.; Fang, B.; Zhang, S. L.; Zhou, C. H. Discovery of Novel Purinylthiazolylethanone Derivatives as Anti-Candida albicans Agents Through Possible Multifaceted Mechanisms. Eur. J. Med. Chem. 2021, 221, 113557; (c) Guo, Y.; Hou, E. H.; Wen, T. Y.; Yan, X. T.; Han, M. Y.; Bai, L. P.; Fu, X. J.; Liu, J. F.; Qin, S. S. Development of Membrane-Active Honokiol/Magnolol Amphiphiles as Potent Antibacterial Agents Against Methicillin-Resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916.
- 24(a) Li, S. R.; Zeng, C. M.; Peng, X. M.; Chen, J. P.; Li, S.; Zhou, C. H. Benzopyrone-mediated Quinolones as Potential Multitargeting Antibacterial Agents. Eur. J. Med. Chem. 2023, 262, 115878; (b) Konai, M. M.; Ghosh, C.; Yarlagadda, V.; Samaddar, S.; Haldar, J. Membrane Active Phenylalanine Conjugated Lipophilic Norspermidine Derivatives with Selective Antibacterial Activity. J. Med. Chem. 2014, 57, 9409–9423.
- 25(a) Gupta, S.; Paul, K. Membrane-active Substituted Triazines Antibacterial Agents Against Staphylococcus aureus with Potential for Low Drug Resistance and Broad Activity. Eur. J. Med. Chem. 2023, 258, 115551; (b) Zhang, J.; Battini, N.; Ou, J. M.; Zhang, S. L.; Zhang, L.; Zhou, C. H. New Efforts toward Aminothiazolylquinolones with Multitargeting Antibacterial Potential. J. Agric. Food Chem. 2023, 71, 2322−2332; (c) Li, W.; Yang, X.; Ahmad, N.; Zhang S. L.; Zhou C. H. Novel Aminothiazoximone-corbelled Ethoxycarbonylpyrimidones with Antibiofilm Activity to Conquer Gram-negative Bacteria Through Potential Multitargeting Effects. Eur. J. Med. Chem. 2024, 268, 116219.
- 26(a) Ibrahim, N. M.; Fahim, S. H.; Hassan, M.; Farag, A. E.; Georgey, H. H. Design and Synthesis of Ciprofloxacin-Sulfonamide Hybrids to Manipulate Ciprofloxacin Pharmacological Qualities: Potency and Side Effects. Eur. J. Med. Chem. 2022, 228, 114021; (b) Zhang, P. L.; Laiche, M. H.; Li, Y. L.; Gao, W. W.; Lin, J. M.; Zhou, C. H. An Unanticipated Discovery of Novel Naphthalimidopropanediols as Potential Broad-Spectrum Antibacterial Members. Eur. J. Med. Chem. 2022, 241, 114657.
- 27(a) Zhu, M. M.; Fang, Y.; Chen, Y. C.; Lei, Y. Q.; Fang, L. F.; Zhu, B. K.; Matsuyama, H. Antifouling and Antibacterial Behavior of Membranes Containing Quaternary Ammonium and Zwitterionic Polymers. J. Colloid Interface Sci. 2021, 584, 225–235; (b) Yang, X. C.; Hu, C. F.; Zhang, P. L.; Li, S.; Hu, C. S.; Geng, R. X.; Zhou, C. H. Coumarin Thiazoles as Unique Structural Skeleton of Potential Antimicrobial Agents. Bioorg. Chem. 2022, 124, 105855; (c) Wang, J.; Ansari, M. F.; Lin, J. M.; Zhou, C. H. Design and Synthesis of Sulfanilamide Aminophosphonates as Novel Antibacterial Agents towards Escherichia coli. Chin. J. Chem. 2021, 39, 2251–2263.
- 28(a) Mancy, A.; Abutaleb, N. S.; Elsebaei, M. M.; Saad, A. Y.; Kotb, A.; Ali, A. O.; Abdel-Aleem, J. A.; Mohammad, H.; Seleem, M. N.; Mayhoub, A. S. Balancing Physicochemical Properties of Phenylthiazole Compounds with Antibacterial Potency by Modifying The Lipophilic Side Chain. ACS Infect. Dis. 2020, 6, 80−90; (b) María, D. A.; Miquel, E.; María-Isabel, M.; Natalia, H.; Rosa, C. D.; Javier, Z.; Ad, F. C.; Michael, T. M.; Daniel, O.; Francesca, B.; Rafael, C. Anti-Biofilm Activity of Murepavadin Against Cystic Fibrosis Pseudomonas aeruginosa Isolates. J. Antimicrob. Chemother. 2021, 76, 2578–2585.
- 29(a) Zhang, N.; Ma, S. T. Recent Development of Membrane-Active Molecules as Antibacterial Agents. Eur. J. Med. Chem. 2019, 184, 111743; (b) Roy, S.; Mondal, A.; Yadav, V.; Sarkar, A.; Banerjee, R.; Sanpui, P.; Jaiswal, A. Mechanistic Insight into The Antibacterial Activity of Chitosan Exfoliated Mos 2 Nanosheets: Membrane Damage, Metabolic Inactivation, and Oxidative Stress. ACS Appl. Bio Mater. 2019, 2, 2738–2755; (c) Yu, J. H.; Xu, X. F.; Hou, W.; Meng, Y.; Huang, M. Y.; Lin, J.; Chen, W. M. Synthetic Cajaninstilbene Acid Derivatives Eradicate Methicillinresistant Staphylococcus aureus Persisters and Biofilms. Eur. J. Med. Chem. 2021, 224, 113691.
- 30(a) Fedorowicz, J.; Cruz, C. D.; Morawska, M.; Ciura, K.; Gilbert-Girard, S.; Mazur, L.; Mäkkylä, H.; Ilina, P.; Savijoki, K.; Fallarero, A.; Tammela, P.; Sączewski, J. Antibacterial and Antibiofilm Activity of Permanently Ionized Quaternary Ammonium Fluoroquinolones. Eur. J. Med. Chem. 2023, 254, 115373; (b) Li, H. X.; Liu, J. Y.; Liu, C. F.; Li, H. Z.; Luo, J. C.; Fang, S. F.; Chen, Y. Z.; Zhong, R. C.; Liu, S. P.; Lin, S. M. Design, Synthesis, and Biological Evaluation of Membrane Active Bakuchiol Derivatives as Effective Broad-Spectrum Antibacterial Agents. J. Med. Chem. 2021, 64, 5603–5619.
- 31(a) Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R. C.; Jin, Y. J.; Wang, W. J.; Li, H. R.; Hou, X. L. Antibacterial Activity and Membrane-Targeting Mechanism of Aloe-Emodin Against Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 621866; (b) Chen, L.; Zhang, J.; Lin, Z.; Zhang, Z. Y.; Mao, M.; Wu, J. C.; Li, Q.; Zhang, Y. Q.; Fan, C. H. Pharmaceutical Applications of Framework Nucleic Acids. Acta Pharm. Sin. B 2022, 12, 76–91.
- 32(a) Ran, B.; Yuan, Y. Y.; Xia, W. X.; Li, M. L.; Yao, Q. C.; Wang, Z. K.; Wang, L. L.; Li, X. Y.; Xu, Y. P.; Peng, X. J. A Photo-Sensitizable Phage for Multidrug-Resistant Acinetobacter baumannii Therapy and Biofilm Ablation. Chem. Sci. 2021, 12, 1054–1061; (b) Cui, S. F.; Addla, D.; Zhou, C. H. Novel 3-Aminothiazolquinolones: Design, Synthesis, Bioactive Evaluation, SARs, and Preliminary Antibacterial Mechanism. J. Med. Chem. 2016, 59, 4488−4510.
- 33(a) Majumder, A.; Sarkar, C.; Das, I.; Sk, S.; Bandyopadhyay, S.; Mandal, S.; Bera, M. Design, Synthesis and Evaluation of a Series of Zinc (II) Complexes of Anthracene-Affixed Multifunctional Organic Assembly as Potential Antibacterial and Antibiofilm Agents Against Methicillin-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2023, 15, 22781–22804; (b) Kong, Q. S.; Li, G. C.; Zhang, F. J.; Yu, T.; Cheng, X. T.; Jing, Q.; Wang, Y. B. N-Arylimidazoliums as Highly Selective Biomimetic Antimicrobial Agents. J. Med. Chem. 2022, 65, 11309–11321.
- 34 Yahia, E.; Mohammad, H.; Abdelghany, T. M.; Fayed, E.; Seleem, M. N.; Mayhoub, A. S. Phenylthiazole Antibiotics: A Metabolism-Guided Approach to Overcome Short Duration of Action. Eur. J. Med. Chem. 2017, 126, 604–613.
- 35(a) Tan, Y. M.; Wang, Y.; Li, S.; Zhang, S. L.; Zhou, C. H. Azolylpyrimidinediols as Novel Structural Scaffolds of DNA-groove Binders Against Intractable Acinetobacter baumannii. J. Med. Chem. 2023, 66, 4910–4931; (b) Sen, S.; Chowdhury, N.; Kim, T. W.; Paul, M.; Debnath, D.; Jeon, S.; Bagchi, A.; Im, J.; Biswas, G. Anticancer, Antibacterial, Antioxidant, and DNA-binding Study of Metal-Phenalenyl Complexes. Bioinorg. Chem. Appl. 2022, 2022, 8453159.
- 36(a) Kuile, B. H.; Hoeksema, M. Antibiotic Killing Through Incomplete DNA Repair. Trends Microbiol. 2018, 26, 2–4; (b) Chen, J. P.; Battini, N.; Ansari, M. F.; Zhou, C. H. Membrane Active 7-Thiazoxime Quinolones as Novel DNA Binding Agents to Decrease The Genes Expression and Exert Potent Antimethicillin-Resistant Staphylococcus aureus Activity. Eur. J. Med. Chem. 2021, 217, 113340.
- 37(a) Ismail, M. M. F.; Abdelkhalek, B. A.; Nossier, E. S.; El Menofy, N. G.; Abdulwahab, H. G. Synthesis of Novel 2-Aminobenzothiazole Derivatives as Potential Antimicrobial Agents with Dual DNA Gyrase/Topoisomerase IV Inhibition. Bioorg. Chem. 2020, 98, 103716; (b) Sun, H.; Huang, S. Y.; Jeyakkumar, P.; Cai, G. X.; Fang, B.; Zhou, C. H. Natural Berberine-derived Azolyl Ethanols as New Structural Antibacterial Agents Against Drug-resistant Escherichia coli. J. Med. Chem. 2022, 65, 436–459.
- 38(a) Chan, P. F.; Srikannathasan, V.; Huang, J. Z.; Cui, H. F.; Fosberry, A. P.; Gu, M. H.; Hann, M. M.; Hibbs, M.; Homes, P.; Ingraham, K.; Pizzollo, J.; Shen, C.; Shillings, A. J.; Spitzfaden, C. E.; Tanner, R.; Theobald, A. J.; Stavenger, R. A.; Bax, B. D.; Gwynn, M. N. Structural Basis of DNA Gyrase Inhibition by Antibacterial QPT-1, Anticancer Drug Etoposide and Moxifloxacin. Nat. Commun. 2015, 6, 10048; (b) He, H.; Xie, M. T.; Zhang, M. T.; Zhang, H. Q.; Zhu, H.; Fang, Y. X.; Shen, Z. H.; Wang, R.; Zhao, Z. J.; Zhu, L. L.; Qian, X. H.; Li, H. L. Design, Synthesis and Biological Evaluation of Potent and Selective S1PR1 Agonists for the Treatment of Ulcerative Colitis. Chin. J. Chem. 2022, 40, 2625–2632.
- 39(a) Kazakova, O.; Lopatina, T.; Giniyatullina, G.; Mioc, M.; Soica, C. Antimycobacterial Activity of Azepanobetulin and Its Derivative: In Vitro, In Vivo, ADMET and Docking Studies. Bioorg. Chem. 2020, 104, 104209; (b) Yang, X. C.; Zhang, P. L.; Kumar, K. V.; Li, S.; Geng, R. X.; Zhou, C. H. Discovery of Unique Thiazolidinone-Conjugated Coumarins as Novel Broad Spectrum Antibacterial Agents. Eur. J. Med. Chem. 2022, 232, 114192; (c) Wang, J.; Battini, N.; Ansari, M. F.; Zhou, C. H. Synthesis and Biological Evaluation of Quinazolonethiazoles as New Potential Conquerors towards Pseudomonas aeruginosa. Chin. J. Chem. 2021, 39, 1093–1103.
- 40(a) Yang, X. C.; Zeng, C. M.; Avula, S. R.; Peng, X. M.; Geng, R. X.; Zhou, C. H. Novel Coumarin Aminophosphonates as Potential Multitargeting Antibacterial Agents Against Staphylococcus aureus. Eur. J. Med. Chem. 2023, 245, 114891; (b) Abid, S. M. A.; Younus, H. A.; Al-Rashida, M.; Arshad, Z.; Maryum, T.; Gilani, M. A. A. I.; Iqbal, J. Sulfonyl Hydrazones Derived From 3-Formylchromone as Non-Selective Inhibitors of MAO-A and MAO-B: Synthesis, Molecular Modelling and In-Silico ADME Evaluation. Bioorg. Chem. 2017, 75, 291–302.
- 41(a) Liu, H. H.; Wang, Y.; Lv, M. X.; Luo, Y.; Liu, B. M.; Huang, Y.; Wang, M.; Wang, J. Y. Flavonoid Analogues as Urease Inhibitors: Synthesis, Biological Evaluation, Molecular Docking Studies and In-silico ADME Evaluation. Bioorg. Chem. 2020, 105, 104370; (b) Zhang, P. L.; Lavanya, G.; Zhang, S. L.; Cai, G. X.; Zhou, C. H. An Unanticipated Discovery towards Novel Naphthalimide Corbelled Aminothiazoximes as Potential Anti-MRSA Agents and Allosteric Modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050; (c) Yu, H. N.; Hou, Z.; Tian, Y.; Mou, Y. H.; Guo, C. Design, Synthesis, Cytotoxicity and Mechanism of Novel Dihydroartemisinin-Coumarin Hybrids as Potential Anti-Cancer Agents. Eur. J. Med. Chem. 2018, 151, 434–449.
- 42 Xie, Y. P.; Ansari, M. F.; Zhang, S. L.; Zhou, C. H. Novel Carbazole-Oxadiazoles as Potential Staphylococcus aureus Germicides. Pestic. Biochem. Physiol. 2021, 175, 104849.
- 43 Wang, J.; Ansari, M. F.; Zhou, C. H. Unique Para-aminobenzenesulfonyl Oxadiazoles as Novel Structural Potential Membrane Active Antibacterial Agents towards Drug-Resistant Methicillin Resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2021, 41, 127995.




