Photocatalytic C(sp3)-H gem-Difluoroallylation and Alkylation with Alkenes via a Base-Assisted Formal 1,2-Hydrogen Atom Transfer of Amidyl Radicals
Meifang Tang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorBingbing Feng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorYanyang Bao
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorZhongtian Xu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorChao Huang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorHanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorGangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Yanan Wang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zheliang Yuan
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMeifang Tang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorBingbing Feng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorYanyang Bao
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorZhongtian Xu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorChao Huang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorHanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorGangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Yanan Wang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zheliang Yuan
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
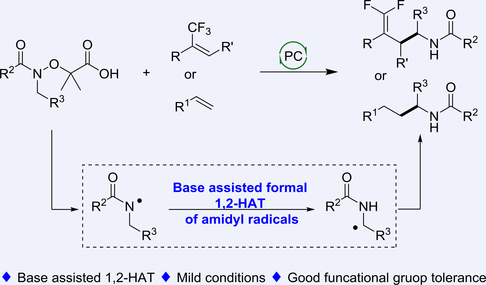
Compared to well-established 1,5-HAT of N-centered radicals, the synthetic applications of 1,2-HAT process were scarce due to the high barrier and constrained three-membered transition state. Here, we have developed a novel C(sp3)-H gem-difluoroallylation via a base assisted formal 1,2-HAT of amidyl radicals with the reductive quenching cycle of photocatalyst. This transformation enables the efficient formation of α-aminoalkyl radicals via 1,2-HAT and showcases good functional group tolerance. Our preliminary mechanistic experiments, along with Density Functional Theory (DFT) calculations demonstrate the feasibility of 1,2-HAT of amidyl radicals, especially when assisted by a base. Furthermore, our method also succeeds in the Giese addition of electron-deficient alkenes as well as styrene.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400122-sup-0001-supinfo.pdfPDF document, 18.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Leitch, J.; Rossolini, T.; Rogova, T.; Maitland, J. A. P.; Dixon, D. J. α-Amino Radicals via Photocatalytic Single-Electron Reduction of Imine Derivatives. ACS Catal. 2020, 10, 2009–2025; (b) Juliá, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352; (c) Hu, J.; Zhu, Z.; Xie, Z.; Le, Z. Recent Advances in Visible-Light-Induced Decarboxylative Coupling Reactions of α-Amino Acid Derivatives. Chin. J. Org. Chem. 2022, 42, 978–1001; (d) Zhao, H.; Cheng, D.; Xu, X. Application of α-Aminoalkyl Radical in Visible Light Catalysis. Chin. J. Org. Chem. 2021, 41, 642–660; (e) Wu, X.; Ren, J.; Shao, Z.; Yang, X.; Qian, D. Transition-Metal-Catalyzed Asymmetric Couplings of α-Aminoalkyl Fragments to Access Chiral Alkylamines. ACS Catal. 2021, 11, 6560–6577; (f) Su, Y.-L.; Doyle, M. P. Application of α-Aminoalkyl Radicals as Reaction Activators. Synthesis 2022, 54, 545–554.
- 2For selected examples, see: (a) McNally, A.; Prier, C. K.; MacMillan, D. W. C. Discovery of an α-Amino C-H Arylation Reaction Using the Strategy of Accelerated Serendipity. Science 2011, 334, 1114–1117; (b) Zhou, W.-J.; Cao, G.-M.; Shen, G.; Zhu, X.-Y.; Gui, Y.-Y.; Ye, J.-H.; Sun, L.; Liao, L.-L.; Li, J.; Yu, D.-G. Visible-light-driven palladium-catalyzed radical alkylation of C–H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 2017, 56, 15683–15687; (c) Li, J.; Kong, M.; Qiao, B.; Lee, R.; Zhao, X.; Jiang, Z. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 2018, 9, 2445; (d) Cauwenbergh, R.; Sahoo, P. K.; Maiti, R. Mathew, A.; Kuniyil, R.; Das, S. Green Chem. 2024, 26, 264–276.
- 3For selected examples, see: (a) Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; Doyle, A. G.; MacMillan, D. W. C. Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl Sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440; (b) Zuo, Z.; Cong, H.; Li, W.; Choi, J.; Fu, G. C.; MacMillan, D. W. C. Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis. J. Am. Chem. Soc. 2016, 138, 1832–1835; (c) Zhang, G.; Zhou, S.; Fu, L.; Chen, P.; Li, Y.; Zou, J.; Liu, G. Asymmetric Coupling of Carbon-Centered Radicals Adjacent to Nitrogen: Copper- Catalyzed Cyanation and Etherification of Enamides. Angew. Chem. Int. Ed. 2020, 59, 20439–20444.
- 4For selected examples, see: (a) Beatty, J. W.; Stephenson, C. R. J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015, 48, 1474–1484; (b) McManus, J. B.; Onuska, N. P. R.; Nicewicz, D. A. Generation and Alkylation of α-Carbamyl Radicals via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2018, 140, 9056–9060; (c) Lahdenperä, A. S. K.; Bacoş, P. D.; Phipps, R. J. Enantioselective Giese Additions of Prochiral α-Amino Radicals. J. Am. Chem. Soc. 2022, 144, 22451–22457; (d) Qin, Y.; Cauwenbergh, R.; Pradhan, S.; Maiti, R.; Franck, P.; Das, S. Nat. Commun. 2023, 14, 7604; (e) Cauwenbergh, R.; Das, S. Photocatalysis: A Green Tool for Redox Reactions. Synlett 2022, 33, 129–149.
- 5For selected examples, see: (a) Remeur, C.; Kelly, C. B.; Patel, N. R.; Molander, G. A. Aminomethylation of Aryl Halides Using α-Silylamines Enabled by Ni/Photoredox Dual Catalysis. ACS Catal. 2017, 7, 6065–6069; (b) Cho, D. W.; Yoon, U. C.; Mariano, P. S. Studies Leading to the Development of a Single-Electron Transfer (SET) Photochemical Strategy for Syntheses of Macrocyclic Polyethers, Polythioethers, and Polyamides. Acc. Chem. Res. 2011, 44, 204–215; (c) Miyazawa, K.; Koike, T.; Akita, M. Hydroaminomethylation of Olefins with Aminomethyltrifluoroborate by Photoredox Catalysis. Adv. Synth. Catal. 2014, 356, 2749–2755.
- 6For selected examples, see: (a) Cheng, W.-M.; Shang, R.; Fu, Y. Photoredox/Brønsted Acid Co-Catalysis Enabling Decarboxylative Coupling of Amino Acid and Peptide Redox-Active Esters with N-Heteroarenes. ACS Catal. 2017, 7, 907–911; (b) Qin, T.; Cornella, J.; Li, C.; Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805; (c) Proctor, R. S. J.; Davis, H. J.; Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 2018, 360, 419–422.
- 7For selected examples, see: (a) Jeffrey, J. L.; Petronijevic, F. R.; MacMillan, D. W. C. Selective Radical–Radical Cross-Couplings: Design of a Formal β-Mannich Reaction. J. Am. Chem. Soc. 2015, 137, 8404–8407; (b) Qi, L.; Chen, Y. Polarity-Reversed Allylations of Aldehydes, Ketones, and Imines Enabled by Hantzsch Ester in Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 13312–13315; (c) de Arriba, A. L. F.; Urbitsch, F.; Dixon, D. J. Umpolung synthesis of branched α-functionalized amines from imines via photocatalytic three-component reductive coupling reactions. Chem. Commun. 2016, 52, 14434–14437; (d) Zhang, T.; Vanderghinste, J.; Guidetti, A.; Doorslaer, S. V.; Barcaro, G.; Monti, S.; Das, S. Angew. Chem. Int. Ed. 2022, 61, e202212083.
- 8For selected examples, see: (a) Ashley, M. A.; Yamauchi, C.; Chu, J. C. K.; Otsuka, S.; Yorimitsu, H.; Rovis, T. Photoredox-catalyzed site-selective α-C(sp3)–H alkylation of primary amine derivatives. Angew. Chem. Int. Ed. 2019, 58, 4002–4006; (b) Shu, X.; Huan, L.; Huang, Q.; Huo, H. Direct Enantioselective C(sp3)-H Acylation for the Synthesis of α-Amino Ketones. J. Am. Chem. Soc. 2020, 142, 19058–19064;. (c) Rand, A. W.; Chen, M.; Montgomery, J. Investigations into mechanism and origin of regioselectivity in the metallaphotoredox- catalyzed α-arylation of N-alkylbenzamides. Chem. Sci. 2022, 13, 10566–10573; (d) Ohmatsu, K.; Suzuki, R.; Furukawa, Y.; Sato, M.; Ooi, T. Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation. ACS Catal. 2020, 10, 2627–2632; (e) Yue, W.-J.; Day, C. S.; Martin, R. Site-Selective Defluorinative sp3 C–H Alkylation of Secondary Amides. J. Am. Chem. Soc. 2021, 143, 6395–6400.
- 9For selected examples, see: (a) Qian, D.; Bera, S.; Hu, X. Chiral Alkyl Amine Synthesis via Catalytic Enantioselective Hydroalkylation of Enecarbamates. J. Am. Chem. Soc. 2021, 143, 1959–1967; (b) He, Y.; Song, H.; Chen, J.; Zhu, S. NiH-catalyzed asymmetric hydroarylation of N-acyl enamines to chiral benzylamines. Nat. Commun. 2021, 12, 638; (c) Cuesta-Galisteo, S.; Schörgenhumer, J.; Wei, X.; Merino, E.; Nevado, C. Nickel-Catalyzed Asymmetric Synthesis of α-Arylbenzamides. Angew. Chem. Int. Ed. 2021, 60, 1605–1609.
- 10(a) Lewis, F. D.; Reddy, G. D. Intramolecular Photochemical Addition Reactions of ω-Styrylaminoalkanes. J. Am. Chem. Soc. 1989, 111, 6465–6466;
(b) Das, S.; Kumar, J. S. D.; Shivaramayya, K.; George, M. V. J. Chem. Soc., Perkin Trans. 1 1995, 1797–1799.
10.1039/P19950001797 Google Scholar
- 11 Zhao, H.; Leonori, D. Minimization of Back-Electron Transfer Enables the Elusive sp3 C–H Functionalization of Secondary Anilines. Angew. Chem. Int. Ed. 2021, 60, 7669–7674.
- 12(a) Bao, Y.; Tang, M.; Wang, Q.; Cao, Z.-Y.; Wang, Y.; Yuan, Z. Visible- Light-Induced Monofluoroalkenylation and gem-Difluoroallylation of Inactivated C(sp3)-H Bonds via 1,5-Hydrogen Atom Transfer (HAT). J. Org. Chem. 2023, 88, 3883–3896; (b) Wang, Q.; Yue, L.; Bao, Y.; Wang, Y.; Gao, Y.; Yuan, Z. Oxalates as Activating Groups for Tertiary Alcohols in Photoredox-Catalyzed gem-Difluoroallylation to Construct All-Carbon Quaternary Centers. J. Org. Chem. 2022, 87, 8237–8247; (c) Li, Y.; Zhang, S.; Wang, Y.; Gao, Y.; Chen, C.; Yuan. Z. Lewis Acid Promoted Vicinal Oxytrifluoromethylselenolation of Alkenes. Org. Lett. 2023, 25, 3210–3215.
- 13(a) Tan, C.-Y.; Kim, M.; Park, I.; Kim, Y.; Hong, S. Site-Selective Pyridine C–H Alkylation with Alcohols and Thiols via Single-Electron Transfer of Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2022, 61, e202213857; (b) Shi, X.; Cao, Y.; Liu, Y.; Niu, K.; Song, H.; Zhang, J.; Wang, Q. Catalyst-free visible-light-induced decarbonylative C–H alkylation of quinoxalin-2(1H)-ones. Org. Chem. Front. 2023, 10, 1296–1300; (c) Rees, M. D.; Hawkins, C. L.; Davies, M. J. Hypochlorite-Mediated Fragmentation of Hyaluronan, Chondroitin Sulfates, and Related N-Acetyl Glycosamines: Evidence for Chloramide Intermediates, Free Radical Transfer Reactions, and Site-Specific Fragmentation. J. Am. Chem. Soc. 2003, 125, 13719–13733; (d) Rees, M. D.; Davies, M. J. Heparan Sulfate Degradation via Reductive Homolysis of Its N-Chloro Derivatives. J. Am. Chem. Soc. 2006, 128, 3085–3097.
- 14During preparation and submission of the manuscript of base assisted formal 1,2-HAT of amidyl radicals generated via oxidative process, Yang and co-workers reported the synthetic application of 1,2-HAT of amidyl radicals generated via reductive process to the synthesis of 1,2-diamine derivatives: (a) Jiang, Y.; Liu, D.; Rotella, M. E.; Deng, G.; Liu, Z.; Chen, W.; Zhang, H.; Kozlowski, M. C.; Walsh, P. J.; Yang, X. Net-1,2-Hydrogen Atom Transfer of Amidyl Radicals: Toward the Synthesis of 1,2-Diamine Derivatives. J. Am. Chem. Soc. 2023, 145, 16045–16057; Besset and co-workers reported α-trifluoromethylthiolation of N-acyl amines via 1,2-HAT of amidyl radicals: (b) Doche, F.; Poisson, T.; Besset, T. Photocatalytic α-Trifluoromethylthiolation of N-Acyl Amines. ACS Catal. 2023, 13, 14112–14120; Chen and co-workers reported visible-light-driven transformation of N-fluoroalkyl hydroxylamine via 1,2-HAT of N-centered radical: (c) Chen, B.; Chen, Q.; Liu, Y.; Chen, J.; Zhou, X.; Wang, H.; Yan, Q.; Wang, W.; Cai, Z.; Chen, F.-E. Visible-Light-Induced Defluorinative α-C(sp3)–H Alkylation for the Synthesis of gem-Difluoroallylated α-Trifluoromethylamines. Org. Lett. 2023, 25, 9124–9129; (d) Liu, Y.; Zhou, T.; Xuan, L.; Lin, Y.; Li, F.; Wang, H.; Lyu, J.; Yan, Q.; Zhou, H.; Wang, W.; Chen, F.-E. Visible-Light-Driven C,N-Selective Heteroarylation of N-Fluoroalkyl Hydroxylamine Reagents with Quinoxalin-2(1H)-ones. Org. Lett. 2023, 25, 8693–8699.
- 15(a) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A. Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586; (b) Guo, W.; Wang, Q.; Zhu, J. Visible light photoredox-catalysed remote C–H functionalisation enabled by 1,5-hydrogen atom transfer (1,5-HAT). Chem. Soc. Rev. 2021, 50, 7359–7377; (c) Zhang, H.; Sun, X.; Ma, C.; Li, C.; Ni, Y.; Yu, Y.; Xu, Y.; Ni, S.; Cao, Z. Copper-Mediated Radical Fluorine-Atom Transfer to Sulfonyl Radical: A Dramatic 4-Methoxypyridine 1-Oxide Ligand Effect. ACS Catal. 2024, 14, 3115–3127.
- 16(a) Zhu, H.; Zheng, H.; Zhang, J.; Feng, J.; Kong, L.; Zhang, F.; Xue, X.-S.; Zhu, G. Solvent-controlled photocatalytic divergent cyclization of alkynyl aldehydes: access to cyclopentenones and dihydropyranols. Chem. Sci. 2021, 12, 11420–1426;
(b) Che, C.; Huang, Q.; Zheng, H.; Zhu, G. Copper-catalyzed cascade annulation of unsaturated α-bromocarbonyls with enynals: a facile access to ketones from aldehydes. Chem. Sci. 2016, 7, 4134–4139;
(c) Elford, P. E.; Roberts, B. P. EPR studies of the formation and transformation of isomeric radicals [C3H5O]˙. Rearrangement of the allyloxyl radical in non-aqueous solution involving a formal 1,2-hydrogen-atom shift promoted by alcohols. J. Chem. Soc., Perkin Trans. 2 1996, 2247–2256;
10.1039/P29960002247 Google Scholar(d) Baciocchi, E.; Bietti, M.; Steenken, S. Base-Catalyzed C−H Deprotonation of 4-Methoxybenzyl Alcohol Radical Cations in Water: Evidence for a Carbon-to-Oxygen 1,2-H-Shift Mechanism. J. Am. Chem. Soc. 1997, 119, 4078–4079; (e) Konya, K. G.; Paul, T.; Lin, S.; Lusztyk, J.; Ingold, K. U. J. Laser Flash Photolysis Studies on the First Superoxide Thermal Source. First Direct Measurements of the Rates of Solvent-Assisted 1,2-Hydrogen Atom Shifts and a Proposed New Mechanism for This Unusual Rearrangement. J. Am. Chem. Soc. 2000, 122, 7518–7527; (f) Fernández-Ramos, A.; Zgierski, M. Z. Theoretical Study of the Rate Constants and Kinetic Isotope Effects of the 1,2-Hydrogen-Atom Shift of Methoxyl and Benzyloxyl Radicals Assisted by Water. J. Phys. Chem. A 2002, 106, 10578–10583; (g) Buszek, R. J.; Sinha, A.; Francisco, J. S. The Isomerization of Methoxy Radical: Intramolecular Hydrogen Atom Transfer Mediated through Acid Catalysis. J. Am. Chem. Soc. 2011, 133, 2013–2015.
- 17(a) Zhang, J.; Liu, D.; Liu, S.; Ge, Y.; Lan, Y.; Chen, Y. Visible- Light-Induced Alkoxyl Radicals Enable α-C(sp3)-H Bond Allylation. iScience 2020, 23, 100755; (b) Liu, D.; Zhang, J.; Chen, Y. Investigations on the 1,2-Hydrogen Atom Transfer Reactivity of Alkoxyl Radicals under Visible-Light-Induced Reaction Conditions. Synlett 2021, 32, 356–361; (c) Shukla, D.; Adiga, S. P.; Ahearn, W. G.; Dinnocenzo, J. P.; Farid, S. Chain Amplified Photochemical Fragmentation of N-Alkoxypyridinium Salts: Proposed Reaction of Alkoxyl Radicals with Pyridine Bases to Give Pyridinyl Radicals. J. Org. Chem. 2013, 78, 1955–1964; (d) Mark, A.; Feinberg, E. C.; Dinnocenzo, J. P. Direct Experimental Evidence for Alkoxyl Radicals Reacting as Hydrogen Atom Donors toward Pyridines. J. Org. Chem. 2021, 86, 7508–7514.
- 18(a) Chen, F.; Xu, X. H.; Chu, L.; Qing, F. L. Visible-Light-Induced Nickel- Catalyzed Radical Cross-Couplings to Access α-Aryl-α-Trifluoromethyl Alcohols. Org. Lett. 2022, 24, 9332–9336; (b) Lombardi, L.; Cerveri, A.; Giovanelli, R.; Castiñeira Reis, M.; Silva López, C.; Bertuzzi, G.; Bandini, M. Direct Synthesis of α-Aryl-α-Trifluoromethyl Alcohols via Nickel Catalyzed Cross-Electrophile Coupling. Angew. Chem. Int. Ed. 2022, 61, e202211732.
- 19 Kim, J. H.; Ruffoni, A.; Al-Faiyz, Y. S. S.; Sheikh, N. S.; Leonori, D. Divergent Strain-Release Amino-Functionalization of [1.1.1] Propellane with Electrophilic Nitrogen-Radicals. Angew. Chem. Int. Ed. 2020, 59, 8225–8231.
- 20 Guo, Y.-Q.; Wu, Y.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Photoredox/Hydrogen Atom Transfer Cocatalyzed C-H Difluoroallylation of Amides, Ethers, and Alkyl Aldehydes. Org. Lett. 2021, 23, 2353–2358.
- 21 Xie, J.; Yu, J.-T.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem. Int. Ed. 2016, 55, 9416–9421.
- 22 Jiang, Y.; Liu, F.; Huang, M.; Luo, X.; Xia, P. Photocatalytic Modular Cyanoalkylamination of Alkenes Involving Two Different Iminyl Radicals. Org. Lett. 2022, 24, 8019–8024.
- 23For recent review on α-trifluoromethyl alkenes, see (a) Zhao, F.; Zhou, W.; Zuo, Z. Recent Advances in the Synthesis of Difluorinated Architectures from Trifluoromethyl Groups. Adv. Synth. Catal. 2022, 364, 234–267; (b) Li, S.; Shu, W. Recent Advances in Radical Enabled Selective Csp3–F Bond Activation of Multifluorinated Compounds. Chem. Commun. 2022, 58, 1066–1077; (c) Tian, F.; Yan, G.; Yu, J. Recent advances in the synthesis and applications of α-(trifluoromethyl)styrenes in organic synthesis. Chem. Commun. 2019, 55, 13486–13505; (d) Jin, Z.; Zhang, F.; Xiao, X.; Wang, N.; Lv, X.; Zhou, L. Recent Advances in N-Heterocyclic Carbene (NHC) Catalyzed Fluorination and Fluoroalkylation. Org. Chem. Front. 2024, 11, 2112–2133.




