Controlling the Reactivity of IBA-N3 by Switching Halogen Salts: Providing a Universal Strategy for Haloazidation of Alkenes
Chen-Xi Xia
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorXin-Lei Sun
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorJinfeng Zhang
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorYue Ren
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorYan Yu
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorKuai Wang
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorCorresponding Author
Ling-Guo Meng
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]Search for more papers by this authorChen-Xi Xia
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorXin-Lei Sun
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorJinfeng Zhang
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorYue Ren
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorYan Yu
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorKuai Wang
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
Search for more papers by this authorCorresponding Author
Ling-Guo Meng
Key Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education; School of Chemistry and Materials Science, Huaibei Normal University, Huaibei, Anhui, 235000 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
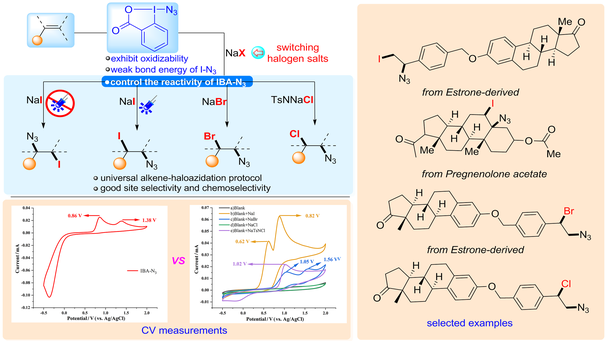
Although various routes have been reported for haloazidation, unavoidable problems exist, such as environmentally unfriendly monomer halogen, the need for in situ generation of unstable halogen azides (XN3), applicability to one type of haloazidation and inability to precisely control selectivity. Herein, we developed a universal strategy for haloazidation of alkenes through controlling the reactivity of IBA-N3 by switching halogen salts, allowing for the synthesis of a diversity of halogen azide products. Mechanistic studies have shown that by tuning the reactivity of IBA-N3 via switching halogen salts, different intermediates can be controllably produced to achieve regioselectivity and chemoselectivity in the haloazidation of alkenes.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400108-sup-0001-supinfo.pdfPDF document, 10.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Beletskaya, I. P.; Cheprakov, A. V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066; (b) Majid, M. H.; Razieh, M.; Masoumeh, M. Recent Advances in the Application of the Heck Reaction in the Synthesis of Heterocyclic Compounds: an Update. Curr. Org. Chem. 2018, 22, 165–198; (c) Zhang, Z.; Qin, Y. Structurally Diverse Poly (thienylene vinylene)s (PTVs) with Systematically Tunable Properties through Acyclic Diene Metathesis (ADMET) and Postpolymerization Modification. Macromolecules 2016, 49, 3318–3327.
- 2(a) Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014, 114, 2587–2693; (b) Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340; (c) McDonald, R. I.; Liu, G.; Stahl, S. S. Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011, 111, 2981–3019; (d) Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Visible-Light-Initiated 4CzIPN Catalyzed Multi-Component Tandem Reactions to Assemble Sulfonated Quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2022, 33, 1479–1482; (e) Yang, S.; Chen, Y.; Ding, Z. Recent Progress of 1,1-Difunctionalization. Org. Biomol. Chem. 2020, 18, 6983–7001; (f) Qin, J.-H.; Nan, N.; Li, J.-H. Electrochemical Difunctionalization of Alkenes. Synthesis 2023, 55, 2843–2859.
- 3(a) Yang, L.; Huang, H. Transition-Metal-Catalyzed direct addition of unactivated C—H bonds to polar unsaturated bonds. Chem. Rev. 2015, 115, 3468–3517; (b) Schultz, D. M.; Wolfe, J. P. Recent Developments in Palladium-Catalyzed Alkene Aminoarylation Reactions for the Synthesis of Nitrogen Heterocycles. Synthesis 2012. 44, 351–361; (c) Giri, R.; KC, S. Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem. 2018, 83, 3013–3022; (d) Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622; (e) Yi, H.; Zhang, G. Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Recent Advances in Radical C–H Activation/Radical Cross-Coupling. Chem. Rev. 2017, 117, 9016–9085; (f) Cheng, J.; Cheng, Y.; Xie, J.; Zhu, C. Photoredox Divergent 1,2-Difunctionalization of Alkenes with gem-Dibromides. Org. Lett. 2017, 19, 6452–6455; (g) Gao, C.; Zeng, J.; Zhang, X.; Liu, Y.; Zhan, Z.-P. A Photosensitizer for N—O Bond Activation: 2,7-Br-4CzIPN-Catalyzed Difunctionalization of Alkenes with Oxime Esters. Org. Lett. 2023, 25, 3146–3151; (h) Shibutani, S.; Nagao, K.; Ohmiya, H. Organophotoredox-Catalyzed Three-Component Coupling of Heteroatom Nucleophiles, Alkenes, and Aliphatic Redox Active Esters. Org. Lett. 2021, 23, 1798–1803; (i) Lv, Y.; Ding, H.; You, J.; Wei, W.; Yi, D. Additive-Free Synthesis of S-Substituted Isothioureas via Visible-Light-Induced Four-Component Reaction of α-Diazoesters, Aryl Isothiocyanates, Amines and Cyclic Ethers. Chin. Chem. Lett. 2024, 35, 109107; (j) Ouyang, W.-T.; Ji, H.-T.; Jiang, J.; Wu, C.; Hou, J.-C.; Zhou, M.-H.; Lu, Y.-H.; Ou, L.-J.; He, W.-M. Ferrocene/Air Double-Mediated FeTiO3-Photocatalyzed Semi-Heterogeneous Annulation of Quinoxalin-2(1H)-ones in EtOH/H2O. Chem. Commun. 2023, 59, 14029–14032; (k) Ji, H.-T.; Wang, K.-L.; Ouyang, W.-T.; Luo, Q.-X.; Lia, H.-X.; He, W.-M. Photoinduced, Additive- and Photosensitizer-Free Multi-Component Synthesis of Naphthoselenazol-2- Amines with Air in Water. Green Chem. 2023, 25, 7983–7987.
- 4(a) Sivaguru, P.; Ning, Y.; Bi, X. New Strategies for the Synthesis of Aliphatic Azides. Chem. Rev. 2021, 121, 4253–4307; (b) Wang, F.; Qi, X.; Liang, Z.; Chen, P.; Liu, G. Copper-Catalyzed Intermolecular Trifluoromethylazidation of Alkenes: Convenient Access to CF3-Containing Alkyl Azides. Angew. Chem. Int. Ed. 2014, 53, 1881–1886; (c) Chen, Z.-M.; Zhang, Z.; Tu, Y.-Q.; Xu, M.-H.; Zhang, F.-M.; Li, C.-C.; Wang, S.-H. A Mn(iii)/TEMPO-co-Mediated Tandem Azidation-1,2- Carbon Migration Reaction of Allylic Silyl Ethers. Chem. Commun. 2014, 50, 10805–10808; (d) Wu, Z.; Ren, R.; Zhu, C. Combination of a Cyano Migration Strategy and Alkene Difunctionalization: The Elusive Selective Azidocyanation of Unactivated Olefins. Angew. Chem. Int. Ed. 2016, 55, 10821–10824; (e) Yuan, Y.-A.; Lu, D.-F.; Chen, Y.-R.; Xu, H. Iron-Catalyzed Direct Diazidation for a Broad Range of Olefins. Angew. Chem. Int. Ed. 2016, 55, 534–538.
- 5(a) Best, M. D. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry 2009, 48, 6571–6584; (b) Sletten, E. M.; Bertozzi, C. R. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676.
- 6 Xi, W.; Scott, T. F.; Kloxin, C. J.; Bowman, C. N. Click Chemistry in Materials Science. Adv. Funct. Mater. 2014, 24, 2572–2590.
- 7(a) Piantadosi, C.; Marasco, C. J.; Morris-Natschke, S. L.; Meyer, K. L.; Gumus, F.; Surles, J. R.; Ishaq, K. S.; Kucera, L. S.; Iyer, N.; Wallen, C. A.; Piantadosi, S.; Modest, E. J. Synthesis and Evaluation of Novel Ether Lipid Nucleoside Conjugates for Anti-HIV-1 Activity. J. Med. Chem. 1991, 34, 1408–1414; (b) Huryn, D. M.; Okabe, M. AIDS-Driven Nucleoside Chemistry. Chem. Rev. 1992, 92, 1745–1768; (c) Pathak, T. Azidonucleosides: Synthesis, Reactions, and Biological Properties. Chem. Rev. 2002, 102, 1623–1667.
- 8(a) Kumar, H. M. S.; Reddy, B. V. S.; Anjaneyulu, S.; Yadav, J. S. A Novel and Efficient Approach to Mono-N-Alkyl Anilines via Addition of Grignard Reagents to Aryl Azides. Tetrahedron Lett. 1999, 40, 8305–8306; (b) Yadav, J. S.; Madhuri, C.; Reddy, B. V. S.; Reddy, G. S. K. K.; Sabitha, G. Indium-Mediated Barbier Reactions of Azides: A Facile Synthesis of N-Allylamine Derivatives. Synth. Commun. 2002, 32, 2771–2777; (c) Kamal, A.; Markandeya, N.; Shankaraiah, N.; Reddy, C. R.; Prabhakar, S.; Reddy, C. S.; Eberlin, M. N.; Santos, L. S. Chemoselective Aromatic Azido Reduction with Concomitant Aliphatic Azide Employing Al/Gd Triflates/NaI and ESI-MS Mechanistic Studies. Chem. Eur. J. 2009, 15, 7215–7224.
- 9(a) Kolb, H. C.; Sharpless, K. B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137; (b) Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979.
- 10 Hantzsch, A. Ueber den Jodstickstoff N3J. Ber. Dtsch. Chem. Ges. 1900, 33, 522–527.
- 11(a) Hassner, A.; Levy, L. A. Additions of iodine azide to olefins. Stereospecific Introduction of Azide Functions. J. Am. Chem. Soc. 1965, 87, 4203–4204; (b) Hassner, A.; Boerwinkle, F. Stereochemistry. XXXIX. Ionic and Free-Radical Addition of Bromine Azide to Olefins. J. Am. Chem. Soc. 1968, 90, 216–218; (c) Spencer, D. A. XXXVII. —The Action of Bromine on Sodium and Silver Azides. J. Chem. Soc., Trans. 1925, 127, 216–224; (d) Terent'ev, A. O.; Krylov, I. B.; Kokorekin, V. A.; Nikishin, G. I. Facile Method for the Synthesis of Vicinal Azidoiodides by the Reaction of the NaN3–I2 System with Unsaturated Compounds. Synth. Commun. 2008, 38, 3797–3809.
- 12(a) Chouthaiwale, P. V.; Karabal, P. U.; Suryavanshi, G.; Sudalai, A. Regiospecific Azidoiodination of Alkenes with Sodium Periodate, Potassium Iodide, and Sodium Azide: A High-Yield Synthesis of β-Iodoazides. Synthesis 2010, 3879–3882;
(b) Cantillo, D.; Gutmann, B.; Kappe, C. O. Safe Generation and Use of Bromine Azide under Continuous Flow Conditions–Selective 1,2-Bromoazidation of Olefins. Org. Biomol. Chem. 2016, 14, 853–857;
(c) Sun, Y.-M.; Yu, L.-Z.; Zhu, Z.-Z.; Hu, X.-B.; Gao, Y.-N.; Shi, M. Electronic Halocyclization and Radical Haloazidation of Benzenelinked 1,7-Dienes for the Synthesis of Functionalized 3,1-Benzoxazines. Org. Biomol. Chem. 2017, 15, 634–639;
(d) Wang, A.-F.; Zhu, Y.-L.; Wang, S.-L.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Metal-Free Radical Haloazidation of Benzene-Tethered 1,7-Enynes Leading to Polyfunctionalized 3,4-Fihydroquinolin-2(1H)- ones. J. Org. Chem. 2016, 81, 1099–1105;
(e) Yan, W.-Q. Lin, M.-Y.; Little, R. D.; Zeng, C.-C. Electrochemical Regioselective Azidoiodination of Alkenes. Tetrahedron 2017, 73, 764–770;
(f) Schulz, G.; George, V.; Taser, D.; Kirschning, A. Taming Bromine Azide for Use in Organic Solvents–Radical Bromoazidations and Alcohol Oxidations. J. Org. Chem. 2023, 88, 3781–3786;
(g) Barluenga, J.; Álvarez-Pérez, M.; Fañanás, F. J.; González, J. M. A Smooth and Practicable Azido-Iodination Reaction of Alkenes Based on IPy2BF4 and Me3SiN3. Adv. Synth. Catal. 2001, 343, 335–337;
(h) Hiraoka, T.; Yano, S.; Hara, S. Iodoazidation of Alkenes by Using Iodine Pentafluoride–Pyridine–Hydrogen Fluoride and Trimethylsilyl Azide. Synthesis 2016, 48, 1353–1358;
(i) Kupracz, L.; Hartwig, J.; Wegner, J.; Ceylan, S.; Kirschning, A. Multistep Flow Synthesis of Vinyl Azides and Their Use in the Copper-Catalyzed Huisgen-Type Cycloaddition under Inductive-Heating Conditions. Beilstein J. Org. Chem. 2011, 7, 1441–1448;
(j) Nair, V.; George, T. G.; Sheeba, V.; Augustine, A.; Balagopal, L.; Nair, L. G. A Novel Regioselective Synthesis of Azidoiodides From Alkenes Using Cerium(IV) Ammonium Nitrate. Synlett 2000, 1597–1598;
(k) Curini, M.; Epifano, F.; Marcotullio, M. C.; Rosati, O. Simple and Regioselective Azidoiodination of Alkenes Using Oxone®. Tetrahedron Lett. 2002, 43, 1201–1203;
(l) Kirschning, A.; Hashem, M. A.; Monenschein, H.; Rose, L.; Schoening, K. U. Preparation of Novel Haloazide Equivalents by Iodine(III)-Promoted Oxidation of Halide Anions. J. Org. Chem. 1999, 64, 6522–6526;
(m) Kirschning, A.; Monenschein, H.; Schmeck, C. Stable Polymer-Bound Iodine Azide. Angew. Chem. Int. Ed. 1999, 38, 2594–2596.
10.1002/(SICI)1521-3773(19990903)38:17<2594::AID-ANIE2594>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 13(a) Achar, T. K.; Maitia, S.; Mal, P. PIDA-I2 Mediated Direct Vicinal Difunctionalization of Olefins: Iodoazidation, Iodoetherification and Iodoacyloxylation, Org. Biomol. Chem. 2016, 14, 4654–4663; (b) Rao, D. S.; Reddy, T. R.; Gurawa, A.; Kumar, M.; Kashyap, S. Photoswitchable Regiodivergent Azidation of Olefins with Sulfonium Iodate(I) Reagent. Org. Lett. 2019, 21, 9990–9994; (c) Saikia, I.; Phukan, P. Facile Generation of Vicinal Bromoazides from Olefins Using TMSN3 and TsNBr2 without Any Catalyst. Tetrahedron Lett. 2009, 50, 5083–5087.
- 14(a) Fumagalli, G.; Rabet, P. T. G.; Boyd, S.; Greaney, M. F. Three- Component Azidation of Styrene-Type Double Bonds: Light-Switchable Behavior of a Copper Photoredox Catalyst. Angew. Chem. Int. Ed. 2015, 54, 11481–11484; (b) Zhu, N.; Wang, F.; Chen, P.; Ye, J.; Liu, G. Copper-Catalyzed Intermolecular Trifluoromethylazidation and Trifluoromethylthiocyanation of Allenes: Efficient Access to CF3-Containing Allyl Azides and Thiocyanates. Org. Lett. 2015, 17, 3580–3583; (c) Ouyang, X.-H.; Song, R.-J.; Liu, Y.; Hu, M.; Li, J.-H. Copper-Catalyzed Radical [2 + 2 + 1] Annulation of Benzene-Linked 1,n-Enynes with Azide: Fused Pyrroline Compounds. Org. Lett. 2015, 17, 6038–6041; (d) Yin, H.; Wang, T.; Jiao, N. Copper-Catalyzed Oxoazidation and Alkoxyazidation of Indoles. Org. Lett. 2014, 16, 2302–2305; (e) Alazet, S.; Preindl, J.; Simonet-Davin, R.; Nicolai, S.; Nanchen, A.; Meyer, T.; Waser, J. Cyclic Hypervalent Iodine Reagents for Azidation: Safer Reagents and Photoredox-Catalyzed Ring Expansion. J. Org. Chem. 2018, 83, 12334–12356.
- 15(a) Zhang, B.; Studer, A. Stereoselective Radical Azidooxygenation of Alkenes. Org. Lett. 2013, 15, 4548–4551; (b) Wang, Y.; Li, G-X.; Yang, G.; He, G.; Chen, G. A Visible-Light-Promoted Radical Reaction System for Azidation and Halogenation of Tertiary Aliphatic C−H Bonds. Chem. Sci. 2016, 7, 2679–2683; (c) Wang, Y.; Hu, X.; Morales-Rivera, C. A.; Li, G-X.; Huang, X.; He, G.; Liu, P.; Chen, G. Epimerization of Tertiary Carbon Centers via Reversible Radical Cleavage of Unactivated C(sp3)-H Bonds. J. Am. Chem. Soc. 2018, 140, 9678–9684.
- 16 Bertho, S.; Rey-Rodriguez, R.; Colas, C.; Retailleau, P.; Gillaizeau, I. Regio- and Stereoselective Iron-Catalyzed Oxyazidation of Enamides using Hypervalent Iodine Reagent. Chem. Eur. J. 2017, 23, 17674–17677.
- 17 Wu, D.; Cui, S.-S.; Lin, Y.; Li, L.; Yu, W. Visible Light-Driven Azidation/Difunctionalization of Vinyl Arenes with Azidobenziodoxole under Copper Catalysis. J. Org. Chem. 2019, 84, 10978–10989.




