Chemodivergent Synthesis of Benzofurans and 2,3-Dihydrobenzofurans via Tandem Oxidative Annulation of Enaminones and Salicylaldehydes
Corresponding Author
Xiyan Duan
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorHui Li
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorJunqi Wang
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorKun Liu
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorMeixin Shi
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorWeidong Lian
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorRan Chen
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorCorresponding Author
Pu Liu
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiyan Duan
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorHui Li
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorJunqi Wang
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorKun Liu
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorMeixin Shi
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorWeidong Lian
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorRan Chen
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
Search for more papers by this authorCorresponding Author
Pu Liu
School of Chemistry & Chemical Engineering, Henan University of Science and Technology, Luoyang, Henan, 471003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
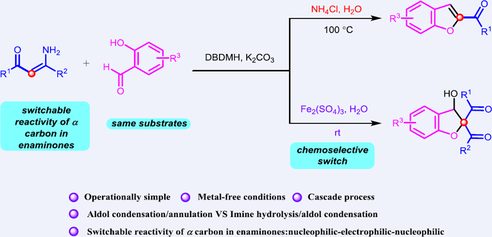
Chemodivergent synthesis of benzofurans and 2,3-dihydrobenzofurans has been realized. Under a reaction system consisting of DBDMH and K2CO3 as promotors, controlled conditions enabled the formation of two sets of valuable heterocycles from the tandem transformation of enaminones and salicylaldehydes. The key to success was the identification of the reaction parameters, in which the imine intermediate which was formed by transient halogenation coupling and substitution processes underwent either aldol condensation/annulation or imine hydrolysis/aldol condensation. The additives NH4Cl or Fe2(SO4)3 controlled the unique selectivity of this reaction. A broad substrate scope of enaminones and salicylaldehydes has been employed in this reaction, demonstrating excellent functional group tolerance and versatility.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400082_sup_0001_supinfo.pdfPDF document, 4.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Schreiber, S. L. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science 2000, 287, 1964−1969; (b) Shenvi, R. A.; O’Malley, D. P.; Baran, P. S. Chemoselectivity: The Mother of Invention in Total Synthesis. Acc. Chem. Res. 2009, 42, 530−541; (c) Kisch, H. Semiconductor Photocatalysis for Chemoselective Radical Coupling Reactions. Acc. Chem. Res. 2017, 50, 1002−1010; (d) Sakakibara, Y.; Murakami, K. Switchable Divergent Synthesis Using Photocatalysis. ACS Catal. 2022, 12, 1857−1878; (e) Tian, H.; Zhang, R.; Shi, L.; Zhao, C.; Wang, X. Chin. J. Chem. 2023, 41, 1783−1790; (f) Xu, W.; Xu, X.; Qing, F. Synthesis and Reactivity of Trifluoromethylthiophosphonium Salts. Chin. J. Chem. 2023, 41, 2779−2787.
- 2(a) Tietze, L. F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115−136; (b) Parsons, P. J.; Penkett, C. S.; Shell, A. J. Tandem Reactions in Organic Synthesis: Novel Strategies for Natural Product Elaboration and the Development of New Synthetic Methodology. Chem. Rev. 1996, 96, 195−206; (c) Hu, L.; Zhang, D.; Huang, X.; Liu, F.; Li, X.; Teng, M.; Huang, G. Metal-Free Arylsulfonyl Radical Triggered Cascade Cyclization of Phenyl-Linked 1,6-Enynes: Synthesis of 2,3-Dihydro-1H-indenes and 10a,11-Dihydro-10H-benzo[b]fluorines. Chin. J. Chem. 2022, 40, 2756−2762.
- 3(a) Ma, C.; Shen, J.; Qu, C.; Shao, T.; Cao, S.; Yin, Y.; Zhao, X.; Jiang, Z. Enantioselective Chemodivergent Three-Component Radical Tandem Reactions through Asymmetric Photoredox Catalysis. J. Am. Chem. Soc. 2023, 145, 20141−20148; (b) Singh, S.; Nerella, S.; Pabbaraja, S.; Mehta, G. Stitching Ynones with Nitromethanes: Domino Synthesis of Functionally Enriched Benzofurans and Benzothiophenes. J. Org. Chem. 2021, 86, 12093−12106.
- 4 Beletskaya, I. P.; Nájera, C.; Yus, M. Chemodivergent reactions. Chem. Soc. Rev. 2020, 49, 7101−7166.
- 5(a) Shamsuzzaman, H. K. Bioactive benzofuran derivatives: a review. Eur. J. Med. Chem. 2015, 97, 483−504; (b) Xu, Z.; Zhao, S.; Lv, Z.; Feng, L.; Wang, Y.; Zhang, F.; Bai, L.; Deng, J. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266−276; (c) Shi, G. Q.; Dropinski, J. F.; Zhang, Y.; Santini, C.; Sahoo, S. P.; Berger, J. P.; MacNaul, K. L.; Zhou, G.; Agrawal, A.; Alvaro, R.; Cai, T.-q.; Hernandez, M.; Wright, S. D.; Moller, D. E.; Heck, J. V.; Meinke, P. T. Novel 2,3-dihydrobenzofuran-2-carboxylic acids: highly potent and subtype-selective PPARα agonists with potent hypolipidemic activity. J. Med. Chem. 2005, 48, 5589–5599; (d) Nichols, D. E.; Hoffman, A. J.; Oberlender, R. A.; Riggs, R. M. Synthesis and Evaluation of 2,3-Dihydrobenzofuran Analogues of the Hallucinogen l-(2,5-Dimethoxy-4-methylphenyI)-2-aminopropane: Drug Discrimination Studies in Rats. J. Med. Chem. 1986, 29, 302–304; (e) Kataoka, K.; Shiota, T.; Takeyasu, T.; Minoshima, T.; Watanabe, K.; Tanaka, H.; Mochizuki, T.; Taneda, K.; Ota, M.; Tanabe, H.; Yamaguchi, H. Potent Inhibitors of Acyl-CoA: Cholesterol Acyltransferase. 2. Structure−Activity Relationships of Novel N-(2,2-Dimethyl-2,3-dihydrobenzofuran- 7-yl)amides. J. Med. Chem. 1996, 39, 1262–1270.
- 6 Loy, N. S. Y.; Singh, A.; Xu, X.; Park, C.-M. Regioselective Synthesis of Benzofuranones and Benzofurans. J. Org. Chem. 2021, 86, 6931−6936.
- 7 Seo, Y. H.; Damodar, K.; Kim, J. K.; Jun, J. G. Synthesis and biological evaluation of 2-aroylbenzofurans, rugchalcones A, B and their derivatives as potent anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2016, 26, 1521–1524.
- 8 Li, Q. B.; Zhou, F. T.; Liu, Z. G.; Li, X. F.; Zhu, W. D.; Xie, J. W. K2CO3-Promoted Domino Reactions: Construction of Functionalized 2,3-Dihydrobenzofurans and Clofibrate Analogues. J. Org. Chem. 2011, 76, 7222–7228.
- 9Review of benzofurans: (a) Dwarakanath, D.; Gaonkar, S. L. Advances in Synthetic Strategies and Medicinal Importance of Benzofurans: A Review. Asian J. Org. Chem. 2022, 11, e202200282; (b) Chiummiento, L.; D’Orsi, R.; Funicello, M.; Lupattelli, P. Last decade of unconventional methodologies for the synthesis of substituted benzofurans. Molecules 2020, 25, 2327; (c) Luca, L. D.; Nieddu, G.; Porcheddu, A.; Giacomelli, G. Some recent approaches to the synthesis of 2-substituted benzofurans. Curr. Med. Chem. 2009, 16, 1–20; (d) Heravi, M. M.; Zadsirjan; Hamidi, H.; Amiri, P. H. T. Total synthesis of natural products containing benzofuran rings. RSC Adv. 2017, 7, 24470; (e) Horton, D. A.; Bourne, G. T.; Smythe, M. L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893; Recent work of benzofurans: (f) Li, Y.; Tung, C. H.; Xu, Z. Synthesis of Benzofuran Derivates via a Gold-Catalyzed Claisen Rearrangement Cascade. Org. Lett. 2022, 24, 5829−5834; (g) Yu, W.; Tung, C. H.; Xu, Z. Synthesis of Benzofurans from Sulfur Ylides and ortho-hydroxy Functionalized Internal Alkynes. Adv. Synth. Catal. 2022, 364, 3749−3753.
- 10(a) Dapkekar, A. B.; Sreenivasulu, C.; Kishore, D. R.; Satyanarayana, G. Recent Advances Towards the Synthesis of Dihydrobenzofurans and Dihydroisobenzofurans. Asian J. Org. Chem. 2022, 11, e202200012; (b) Laurita, T.; D’Orsi, R.; Chiummiento, L.; Funicello, M.; Lupattelli, P. Recent Advances in Synthetic Strategies to 2,3-Dihydrobenzofurans. Synthesis 2020, 52, 1451−1477.
- 11(a) Schevenels, F.; Markó, I. E. Metal-Free One-Pot Synthesis of 3-Phosphinoylbenzofurans via Phospha-Michael Addition/Cyclization of H-Phosphine Oxides and in situ Generated ortho-Quinone Methides. Org. Lett. 2018, 20, 477−480; (b) Sivaraman, A.; Harmalkar, D.; Kang, J.; Choi, Y.; Lee, K. A protecting group-free divergent synthesis of natural benzofurans via one-pot synthesis of 2-bromo-6-hydroxybenzofurans. Org. Biomol. Chem. 2019, 17, 2153−2161; (c) Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y.; Ye, F.; Zhang, Y.; Wang, J. CuBr-Catalyzed Coupling of N-Tosylhydrazones and Terminal Alkynes: Synthesis of Benzofurans and Indoles. Org. Lett. 2011, 13, 968−971; (d) Moure, M. J.; SanMartin, R.; Dominguez, E. Benzofurans from Benzophenones and Dimethylacetamide: Copper-Promoted Cascade Formation of Furan O1-C2 and C2-C3 Bonds Under Oxidative Conditions. Angew. Chem. Int. Ed. 2012, 51, 3220–3224; (e) Wang, J.-R.; Manabe, K. Hydroxyterphenylphoshine−Palladium Catalyst for Benzo[b]furan Synthesis from 2-Chlorophenols. Bifunctional Ligand Strategy for Cross-Coupling of Chloroarenes. J. Org. Chem. 2010, 75, 5340–5342.
- 12Reviews: (a) Xia, X. F.; Niu, Y. N. Recent developments in the synthesis of nitrogen-containing heterocycles from β-aminovinyl esters/ketones as C=C-N donors. Org. Biomol. Chem. 2022, 20, 282–295; (b) Chen, X. Y.; Zhang, X. T.; Wan, J.-P. Recent advances in transition metal-free annulation toward heterocycle diversity based on the C–N bond cleavage of enaminone platform. Org. Biomol. Chem. 2022, 20, 2356; (c) Wang, Z.; Zhao, B.; Liu, Y. Y.; Wan, J.-P. Recent Advances in Reactions Using Enaminone in Water or Aqueous Medium. Adv. Synth. Catal. 2022, 364, 1508–1521; (d) Poulsen, T. B. Total Synthesis of Natural Products Containing Enamine or Enol Ether Derivatives. Acc. Chem. Res. 2021, 54, 1830−1842; (e) Stanovnik, B. Enaminone, Enaminoesters, and Related Compounds in the Metal-Free Synthesis of Pyridines and Fused Pyridines. Eur. J. Org. Chem. 2019, 5120–5132; (f) Fu, L.; Wan, J.-P. C3-Functionalized Chromones Synthesis by Tandem C−H Elaboration and Chromone Annulation of Enaminones. Asian J. Org. Chem. 2019, 8, 767–776; (g) Wan, J.-P.; Gao, Y. Domino Reactions Based on Combinatorial Bond Transformations in Electron-Deficient Tertiary Enamines. Chem. Rec. 2016, 16, 1164–1177; (h) Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480; (i) Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J. P. Recent advances in visible light-me diated chemical transformations of enaminones. Chin. Chem. Lett. 2024, 35, 108977; (j) Ye, J.; Liu, Y.; Luo, J.; Wan, J. P. “Alkene-to-Alkene” Difunctionalization of Enaminones for the Synthesis of Polyfunctionalized Alkenes by Transition-Metal-Free C−H and C−N Bond Transformation. Org. Lett. 2023, 25, 8451−8456; (k) Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Yan, S.; Yu, F. Rh(III)-Catalyzed [3 + 2] Annulation/Pinacol Rearrangement Reaction of Enaminones with Iodonium Ylides: Direct Synthesis of 2-Spirocyclo-pyrrol-3-ones. Org. Lett. 2023, 25, 7214−7219; (l) Zhong, Z.; Liao, L.; Liu, Y.; Zhang, M.; Wan, J. P. Annulation of enaminones with quinonediimides/quinoneimides for selective synthesis of indoles and 2-aminobenzofurans. Chem. Commun. 2023, 59, 6885; (m) Hang, L.; Liu, Y.; Wan, J. P. Engineering Biomass Feedstock Cyrene to Value-Added Compounds by Enaminone Platform Construction. Chin. J. Org. Chem. 2023, 43, 2096–2103; (n) Ashitha, K. T.; Krishna, M. S. A.; Basavaraja, D.; Somappa, S. Org. Chem. Front. 2022, 9, 5306–5358; (o) Chen, D.; Jiang, J.; Wan, J. P. Advances in the Transition Metal-Free C—H Trifluoromethylation. Chin. J. Chem. 2022, 40, 2582–2594.
- 13(a) Duan, X. Y.; Liu, X.; Cuan, X.; Wang, L.; Liu, K.; Zhou, H.; Chen, X.; Li, H.; Wang, J. Solvent-Controlled Synthesis of Thiocyanated Enaminones and 2-Aminothiazoles from Enaminones, KSCN, and NBS. J. Org. Chem. 2019, 84, 12366−12376; (b) Duan, X. Y.; Li, H.; Li, W.; Wang, J.; Liu, N. NBS-Promoted C−H Amination of Enaminones for the Synthesis of N-Heterocycle Substituted Enaminones. ChemistrySelect 2021, 6, 6478–6482; (c) Duan, X. G.; Liu, K.; Meng, Z.; Guo, Y.; Li, H.; Liu, N.; Qu, W.; Duan, X. Y.; Ma, J. 1,3-Dibromo-5,5-dimethylhydantoin (DBDMH)-promoted cross-coupling of enaminones with phenols under metal-free conditions. Tetrahedron Lett. 2022, 107, 154111; (d) Liu, N.; Cuan, X.; Li, H.; Duan, X. Chin. J. Org. Chem. 2023, 43, 602−621.




