Total Synthesis and Stereochemical Assignment of Talaroconvolutin A and Talarofuranone: Gram-scale Synthesis of Ferroptosis Inducer Talaroconvolutin A
Ming Yao
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorWei Yang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorJing Li
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorChengyun Huang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorJin Fang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorSulu Qin
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorShuzhi Liu
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Xiaolong Yang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
*E-mail: [email protected]Search for more papers by this authorMing Yao
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorWei Yang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorJing Li
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorChengyun Huang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorJin Fang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorSulu Qin
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorShuzhi Liu
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Xiaolong Yang
School of Pharmaceutical Sciences, South-Central Minzu University, Wuhan, Hubei, 430074 China
*E-mail: [email protected]Search for more papers by this authorComprehensive Summary
The first total synthesis of talaroconvolutin A (1.1 g obtained) and talarofuranone has been achieved using accessible materials (12 steps, 7.5% and 8.2% yields, respectively). Convergent routes involved intramolecular Diels−Alder reactions in two approaches for creating the decalin moiety. Additionally, an unprecedented DMP-mediated domino reaction resulted in the deoxy-tetramic acid system. These syntheses not only establish the absolute configuration of talaroconvolutin A but also enable further collaborative studies of this type of ferroptosis inducers.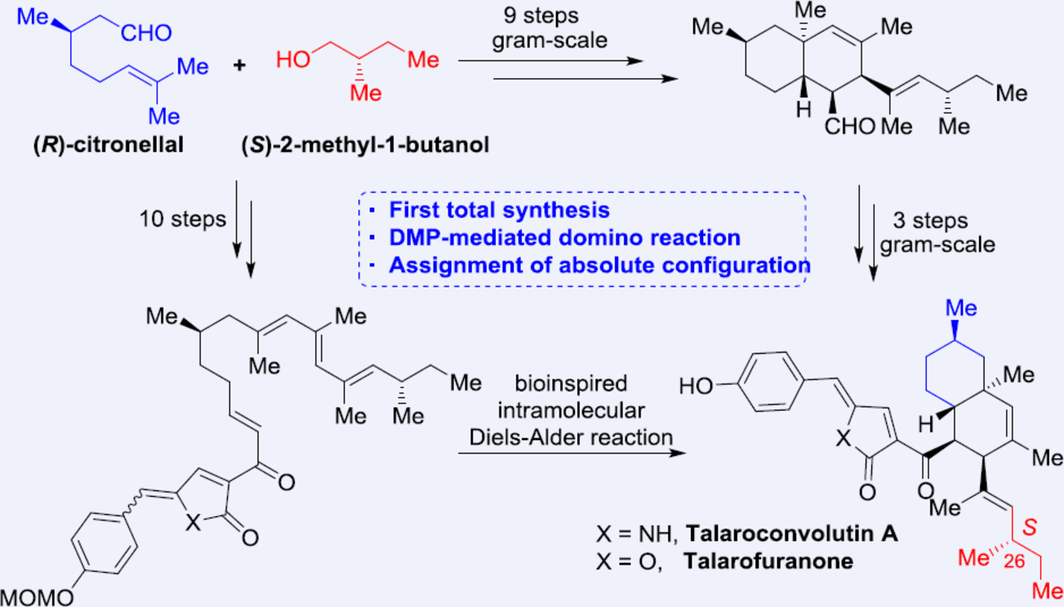
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400061-sup-0001-supinfo.pdfPDF document, 7.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Sun, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- 2(a) Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil Resistance Mechanisms in Colorectal Cancer: From Classical Pathways to Promising Processes. Cancer Sci. 2020, 111, 3142–3154; (b) Dekker, E.; Tanis, P. J.; Vleugels, J. L. A.; Kasi, P. M.; Wallace, M. B. Colorectal Cancer. Lancet 2019, 394, 1467–1480; (c) Hammond, W. A.; Swaika, A.; Mody, K. Pharmacologic Resistance in Colorectal Cancer: A Review. Ther. Adv. Med. Oncol. 2016, 8, 57–84.
- 3(a) Liang, H.; He, X.; Tong, Y.; Bai, N.; Pu, Y.; Han, K.; Wang, Y. Ferroptosis Open a New Door for Colorectal Cancer Treatment. Front. Oncol. 2023, 13, 1059520; (b) Koeberle, S. C.; Kipp, A. P.; Stuppner, H.; Koeberle, A. Ferroptosis-Modulating Small Molecules for Targeting Drug-Resistant Cancer: Challenges and Opportunities in Manipulating Redox Signaling. Med. Res. Rev. 2023, 43, 614–616; (c) Yan, H.; Talty, R.; Aladelokun, O.; Bosenberg, M.; Johnson, C. H. Ferroptosis in Colorectal Cancer: A Future Target? Brit. J. Cancer 2023, 128, 1439–1451; (d) Wang, Y.; Zhang, Z.; Sun, W.; Zhang, J.; Xu, Q.; Zhou, X.; Mao, L. Ferroptosis in Colorectal Cancer: Potential Mechanisms and Effective Therapeutic Targets. Biomed. Pharmacother. 2022, 153, 113524; (e) Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in Cancer Therapy: A Novel Approach to Reversing Drug Resistance. Mol. Cancer 2022, 21, 47; (f) Li, F.; Long, H.; Zhou, Z.; Luo, H.; Xu, S.; Gao, L. System Xc−/GSH/GPX4 Axis: An Important Antioxidant System for the Ferroptosis in Drug-Resistant Solid Tumor Therapy. Front. Pharmacol. 2022, 13, 910292; (g) Liang, X.; You, Z.; Chen, X.; Li, J. Targeting Ferroptosis in Colorectal Cancer. Metabolites 2022, 12, 745.
- 4 Suzuki, S.; Hosoe, T.; Nozawa, K.; Kawai, K.; Yaguchi, T.; Udagawa, S. Antifungal Substances Against Pathogenic Fungi, Talaroconvolutins, from Talaromyces convolutus. J. Nat. Prod. 2000, 63, 768–772.
- 5(a) Xia, Y.; Xiang, L.; Yao, M.; Ai, Z.; Yang, W.; Guo, J.; Fan, S.; Liu, N.; Yang, X. Proteomics, Transcriptomics, and Phosphoproteomics Reveal the Mechanism of Talaroconvolutin A Suppressing Bladder Cancer via Blocking Cell Cycle and Triggering Ferroptosis. Mol. Cell. Proteomics 2023, 22, 100672; (b) Xia, Y.; Liu, S.; Li, C.; Ai Z.; Shen, W.; Ren, W.; Yang, X. Discovery of a Novel Ferroptosis Inducer-Talaroconvolutin A–Killing Colorectal Cancer Cells in vitro and in vivo. Cell Death Dis. 2020, 11, 988.
- 6 Wang, P.; Cui, Y.; Cai, C.; Chen, H.; Dai, Y.; Chen, P.; Kong, F.; Yuan, J.; Song, X.; Mei, W.; Dai, H. Two New Succinimide Derivatives Cladosporitins A and B from the Mangrove-Derived Fungus Cladosporium sp. HNWSW-1. Mar. Drugs 2019, 17, 4.
- 7(a) Neubert, B. J. Total Synthesis of (±)-Phloeodictine A1, a Novel Biomimetic Route to the 3-Acyl-5-hydroxy-3-pyrrolin-2-one and 3-Acyl-3,4-epoxy-5-hydroxypyrrolidin-2-one Ring Systems and Syntheses of Ficuseptine, Juliprosine, and Juliprosopine. Ph.D. Dissertation, University of Brandeis, 2005; (b) Snider, B. B.; Neubert, B. J. A Novel Biomimetic Route to the 3-Acyl-5-hydroxy-3-pyrrolin-2-one and 3-Acyl-3,4-epoxy-5-hydroxypyrrolidin-2-one Ring Systems. J. Org. Chem. 2004, 69, 8952–8955.
- 8Shionozaki, N.; Iwamura, N.; Tanaka, R.; Makino, K.; Uchiro, H. Total Synthesis of Diaporthichalasin by Using the Intramolecular Diels–Alder Reaction of an α,β-Unsaturated γ-Hydroxylactam in Aqueous Media. Chem. Asian J. 2013, 8, 1243–1252; (b) Shionozaki, N.; Yamaguchi, T.; Kitano, H.; Tomizawa, M.; Makino, K.; Uchiro, H. Total Synthesis of Myceliothermophins A-E. Tetrahedron Lett. 2012, 53, 5167–5170.
- 9 Nicolaou, K. C.; Shi, L.; Lu, M.; Pattanayak, M. R.; Shah, A. A.; Ioannidou, H. A.; Lamani, M. Total Synthesis of Myceliothermophins C, D, and E. Angew. Chem. Int. Ed. 2014, 53, 10970–10974.
- 10(a) Liu, X.; Qin, Y. Industrial Total Synthesis of Natural Medicines. Nat. Prod. Rep. 2023, 40, 1694–1700; (b) Kuttruff, C. A.; Eastgate, M. D.; Baran, P. S. Natural Product Synthesis in the Age of Scalability. Nat. Prod. Rep. 2014, 31, 419–432.
- 11(a) Paul, D.; Kundu, A.; Saha, S.; Goswami, R. K. Total Synthesis: The Structural Confirmation of Natural Products. Chem. Commun. 2021, 57, 3307–3322; (b) Menna, M.; Imperatore, C.; Mangoni, A.; Sala, G. D.; Taglialatela-Scafati, O. Challenges in the Configuration Assignment of Natural Products. A Case-Selective Perspective. Nat. Prod. Rep. 2019, 36, 476–489; (c) Nicolaou, K. C.; Snyder, S. A. Chasing Molecules That Were Never There: Misassigned Natural Products and the Role of Chemical Synthesis in Modern Structure Elucidation. Angew. Chem. Int. Ed. 2005, 44, 1012–1044.
- 12 Truax, N. J.; Romo, D. Bridging the Gap Between Natural Product Synthesis and Drug Discovery. Nat. Prod. Rep. 2020, 37, 1436–1453.
- 13For reviews about the total synthesis of natural products with a tetramic acid motif see: (a) Zhang, W.; Kaplan, A. R.; Davison, E.; Freeman, J. L.; Brimble, M. A.; Wuest, W. M. Building trans-Bicyclo[4.4.0]decanes/decenes in Complex Multifunctional Frameworks: The Case for Antibiotic Development. Nat. Prod. Rep. 2021, 38, 880–889; (b) Li, X.; Zhang, W.; Gao, S. Total Synthesis of Complex Natural Products: Combination of Chemical Synthesis and Biosynthesis Strategies. Chin. J. Org. Chem. 2018, 38, 2185–2198; (c) Dhambri, S.; Mohammad, S.; Nguyen Van Buu, O.; Galvani, G.; Meyer, Y.; Lannou, M.-I.; Sorin, G.; Ardisson, J. Recent Advances in the Synthesis of Natural Multifunctionalized Decalins. Nat. Prod. Rep. 2015, 32, 841–864; Total synthesis of equisetin: (d) Yin, J.; Kong, L.; Wang, C.; Shi, Y.; Cai, S.; Gao, S. Biomimetic Synthesis of Equisetin and (+)-Fusarisetin A. Chem. Eur. J. 2013, 19, 13040–13046; (e) Yuki, K.; Shindo, M.; Shishido, K. Enantioselective Total Synthesis of (–)-Equisetin Using a Me3Al-mediated Intramolecular Diels-Alder Reaction. Tetrahedron Lett. 2001, 42, 2517–2519; (f) Burke, L. T.; Dixon, D. J.; Ley, S. V.; Rodriguez, F. A Short Stereoselective Total Synthesis of the Fusarium Toxin Equisetin. Org. Lett. 2000, 2, 3611–3613; Total synthesis of fusarisetin A: (g) Liu, C.; Zeng, Z.; Chen, R.; Jiang, X.; Wang, Y.; Zhang, Y. Total Synthesis of (+)-Fusarisetin A Driven by a One-pot Four-reaction Process. Org. Lett. 2016, 18, 624–627; (h) Xu, J.; Caro-Diaz, E. J. E.; Trzoss, L.; Theodorakis, E. A. Nature-inspired Total Synthesis of (-)-Fusarisetin A. J. Am. Chem. Soc. 2012, 134, 5072–5075; (i) Xu, J.; Caro-Diaz, E. J. E.; Lacoske, M. H.; Hung, C.-I.; Jamora, C.; Theodorakis, E. A. Fusarisetin A: scalable total synthesis and related studies. Chem. Sci. 2012, 3, 3378–3386; (j) Deng, J.; Zhu, B.; Lu, Z.; Yu, H.; Li, A. Total Synthesis of (–)-Fusarisetin A and Reassignment of the Absolute Configuration of Its Natural Counterpart. J. Am. Chem. Soc. 2012, 134, 920–923.
- 14(a) Lichman, B. R.; O’Connor, S. E.; Kries, H. Biocatalytic Strategies Towards [4+2] Cycloadditions. Chem. Eur. J. 2019, 25, 6864–6877; (b) Li, X.; Zheng, Q.; Yin, J.; Liu, W.; Gao, S. Chem-enzymatic Synthesis of Equisetin. Chem. Commun. 2017, 53, 4695–4697; (c) Sims, J. W.; Fillmore, J. P.; Warner, D. D.; Schmidt, E. W. Equisetin Biosynthesis in Fusarium Heterosporum. Chem. Commun. 2005, 186–188; (d) Wang, X.; Zhao, L.; Liu, C.; Qi, J.; Zhao, P.; Liu, Z.; Li, C.; Hu, Y.; Yin, X.; Liu, X.; Liao, Z.; Zhang, L.; Xia, X. New Tetramic Acids Comprising of Decalin and Pyridones from Chaetomium Olivaceum SD-80A with Antimicrobial Activity. Front. Microbiol. 2020, 10, 2958; (e) Nay, B.; Riache, N.; Evanno, L. Chemistry and Biology of Non-tetramic γ-hydroxy-γ-lactams and γ-Alkylidene-γ-lactams from Natural Sources. Nat. Prod. Rep. 2009, 26, 1044–1062.
- 15Eade, S. J.; Walter, M. W.; Byrne, C.; Odell, B.; Rodriguez, R.; Baldwin, J. E.; Adlington, R. M.; Moses, J. E. Biomimetic Synthesis of Pyrone-Derived Natural Products: Exploring Chemical Pathways from a Unique Polyketide Precursor. J. Org. Chem. 2008, 73, 4830–4839; (b) Compound 7 is unstable and must be prepared prior to use.
- 16 Jessen, H. J.; Schumacher, A.; Shaw, T.; Pfaltz, A.; Gademann, K. A Unified Approach for the Stereoselective Total Synthesis of Pyridone Alkaloids and Their Neuritogenic Activity. Angew. Chem. Int. Ed. 2011, 50, 4222–4226.
- 17(a) Choy, P. Y.; Gan, K. B.; Kwong, F. K. Recent Expedition in Pd-Catalyzed Sonogashira Coupling and Related Processes. Chin. J. Chem. 2023, 41, 1099–1118; (b) Arcadi, A.;. Burini, A.; Cacchi, S.; Delmastro, M.; Marinelli, F.; Pietroni, B. R. Palladium-Catalyzed Reaction of Vinyl Triflates and Vinyl/Aryl Halides with 4-Alkynoic Acids: Regio- and Stereoselective Synthesis of (E)-δ-Vinyl/Aryl-γ-methylene- γ-butyrolactones. J. Org. Chem. 1992, 57, 976–982; (c) The E configuration of the double bond of compound 10 was determined by an NOESY experiment.
- 18For the conversion of (E)-lactone to (Z)-lactam see: (a) O’Neal, W. G.; Roberts, W. P.; Ghosh, I.; Jacobi, P. A. Studies in Chlorin Chemistry. II. A Versatile Synthesis of Dihydrodipyrrins. J. Org. Chem. 2005, 70, 7243–7251; (b) Sam, J.; Lopez, A. V.; Shafik, R. M. Preparation and Properties of Some Relatives of Noscapine. J. Pharm. Sci. 1968, 57, 1755–1759; For recent reports about the synthesis of pyrrolinone see: (c) Liu, D.; Song, S.; Chen, L.; Zhang, M.; Liu, Z.; Lu, X.; Huang, J.; Yu, F. Access to Thiionized-, Selenolized-, and Alkylated 5-alkylidene 3-pyrrolin-2-one Derivatives via a Regioselective Oxidative Annulation Reaction. Org. Biomol. Chem. 2023, 21, 2596–2602; (d) Huang, X.; Yu, J.; Luan, X. [3+2] Cycloaddition of Vinyl Cyclopropane and Hydroxylamines via Isocynate Intermediate to γ-Lactams. Chin. J. Chem. 2023, 41, 1937–1942; (e) Caruano, J.; Mucciolib, G. G.; Robiette, R. Biologically Active γ-Lactams: Synthesis and Natural Sources. Org. Biomol. Chem. 2016, 14, 10134–10156.
- 19(a) Watanabe, Y.; Morozumi, H.; Mutoh, H.; Hagiwara, K.; Inoue, M. Total Synthesis of (-)-Batrachotoxin Enabled by a Pd/Ag-Promoted Suzuki-Miyaura coupling reaction. Angew. Chem. Int. Ed. 2023, e202309688; (b) Ohyoshi, T.; Iizumi, H.; Hosono, S.; Tano, H.; Kigoshi, H. Total Synthesis of Aplysiasecosterols A and B, Two Marine 9,11-Secosteroids. Org. Lett. 2023, 25, 4725–4729.
- 20(a) Gnaim, S.; Vantourout, J. C.; Serpier, F.; Echeverria, P.-G.; Baran, P. S. Carbonyl Desaturation: Where Does Catalysis Stand? ACS Catal. 2021, 11, 883–892; (b) Nicolaou, K. C.; Zhong, Y.; Baran, P. S. A New Method for the One-Step Synthesis of α,β-Unsaturated Carbonyl Systems from Saturated Alcohols and Carbonyl Compounds. J. Am. Chem. Soc. 2000, 122, 7596–7597.
- 21(a) The relative configuration of (19R,26S)-1 deduced from NOESY experiments (Figure S1), proved to be the same as those in natural talaroconvolutin A. The stereochemistry of the double bond between C5 and C6 was determined to be in the Z configuration based on the key correlation H-4/H-6, in the NOESY spectrum. For more details see SI; (b) Cui, C.; Dai, M. Total Synthesis of UCS1025A via Tandem Carbonylative Stille Cross Coupling and Diels−Alder Reaction. Chin. J. Chem. 2023, 41, 3019–3024.
- 22(a) Tian, P.; Ye, W.; Zhang, X.; Tong, Y.; Qian, P.; Tong, R. Ten-step Asymmetric Total Synthesis of Potent Antibiotics Anthracimycin and Anthracimycin B. Chem. Sci. 2022, 13, 12776–12781; (b) Meguro, Y.; Ito, J.; Nakagawa, K.; Kuwahara, S. Total Synthesis of the Broad-Spectrum Antibiotic Amycolamicin. J. Am. Chem. Soc. 2022, 144, 5253–5257; (c) Fu, Q.; Wang, Y.; Nan, F. Construction of the Hexacyclic Core of Dispirocochlearoids A—C via a Diels−Alder Reaction. Chin. J. Chem. 2022, 40, 1566–1570; (d) Davison, E. K.; Freeman, J. L.; Zhang, W.; Wuest, W. M.; Furkert, D. P.; Brimble, M. A. Asymmetric Total Synthesis of the Naturally Occurring Antibiotic Anthracimycin. Org. Lett. 2020, 22, 5550–5554.
- 232D NOESY analysis showed correlations between H-2 and H-18, and between H-1, H-20, and H-10, with no correlations between H-3 and H-10, and between H-10 and H-18, further suggesting the formation of trans-decalin products (Figure S2).
- 24(a) Attempts to prepare compound 16 by oxidation of compound 15 with other oxidizing agents including IBX, TPAP/NMO, and SO3·Py, were unsuccessful; (b) For water and pyridine promoted DMP oxidation reaction see: Meyer, S. D.; Schreiber, S. L. Acceleration of the Dess-Martin Oxidation by Water. J. Org. Chem. 1994, 59, 7549–7552.
- 25The conversion and reproducibility of the reaction are greatly influenced by the ratio of DMP, water and pyridine. After screening the amounts of water and pyridine, we found that the combination of DMP (5.5 equiv.), water (6.05 equiv.) and pyridine (11.0 equiv.) resulted in a complete conversion of the substrate in 2 h at room temperature, with a high yield and reproducibility.
- 26 Padmathilake, K. G. E.; Bandara, H. M. S. K. H.; Mallique Qader, M.; Savitri Kumar, N.; Jayasinghe, L.; Masubuti, H.; Fujimoto, Y. Talarofuranone, a New Talaroconvolutin Analog from the Endophytic Fungus Talaromyces purpurogenus from Pouteria Campechiana Seeds. Nat. Prod. Commun. 2017, 12, 489–490.




