Encouraging Solution to the Problem of Synthesizing Protein α-Thioester
Xinliang Liu
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorZijun Gao
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorJie Zhao
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorFarong Ye
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Ping Huang
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ping Wang
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Shenzhen Research Institute of Shanghai Jiao Tong University, Shenzhen, Guangdong, 518057 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXinliang Liu
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorZijun Gao
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorJie Zhao
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorFarong Ye
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Ping Huang
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ping Wang
Center for Chemical Glycobiology, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai, 200240 China
Shenzhen Research Institute of Shanghai Jiao Tong University, Shenzhen, Guangdong, 518057 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
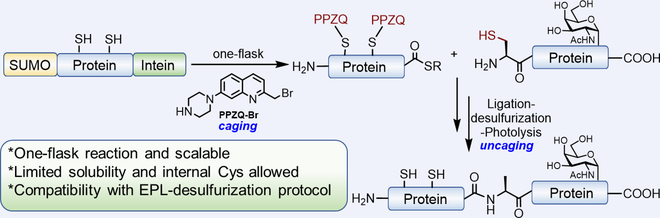
Expressed protein ligation (EPL) provides a powerful tool to access large-size proteins with precise structures. Existing methods for constructing the critical protein thioester for EPL have predominantly relied on the recombinant intein fusion expressed in Escherichia coli (E. coli). Despite its powerful applications, the expression of thioester derived from eukaryotic protein in E. coli inherently suffers from its limited solubility, the inactivity of intein, premature hydrolysis and low yields. To overcome these obstacles, we present herein the facile one-flask synthesis of inaccessible protein α-thioester via a SUMO-protein-intein (SPI) sandwich model. The utility of SUMO enhances the protein fusion yield and solubility, prevents premature hydrolysis and simplifies the purification process. The inaccessible protein thioester with internal Cys residues can be readily produced and is compatible with the EPL-desulfurization protocol used to prepare complex proteins, which is otherwise difficult to obtain using traditional methods. Its utility has been highlighted through the synthesis of human granulocyte colony-stimulating factor (G-CSF).
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300762-sup-0001-Supinfo.pdfPDF document, 3.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Agouridas, V.; El Mahdi, O.; Diemer, V.; Cargoët, M.; Monbaliu, J. C. M.; Melnyk, O. Native Chemical Ligation and Extended Methods: Mechanisms, Catalysis, Scope, and Limitations. Chem. Rev. 2019, 119, 7328–7443; (b) Bondalapati, S.; Jbara, M.; Brik, A. Expanding the chemical toolbox for the synthesis of large and uniquely modified proteins. Nat. Chem. 2016, 8, 407–418; (c) Flood, D. T.; Hintzen, J. C. J.; Bird, M. J.; Cistrone, P. A.; Chen, J. S.; Dawson, P. E. Leveraging the Knorr Pyrazole Synthesis for the Facile Generation of Thioester Surrogates for use in Native Chemical Ligation. Angew. Chem. Int. Ed. 2018, 57, 11634–11639; (d) Li, Y. X.; Liu, J. Z.; Zhou, Q. Q.; Zhao, J.; Wang, P. Preparation of Peptide Selenoesters from Their Corresponding Acyl Hydrazides. Chin. J. Chem. 2021, 39, 1861–1866; (e) Wang, S. Y.; Thopate, Y. A.; Zhou, Q. Q.; Wang, P. Chemical Protein Synthesis by Native Chemical Ligation and Variations Thereof. Chin. J. Chem. 2019, 37, 1181–1193.
- 2 Severinov, K.; Muir, T. W. Expressed Protein Ligation, a Novel Method for Studying Protein-Protein Interactions in Transcription. J. Biol. Chem. 1998, 273, 16205–16209.
- 3(a) Ai, H. S.; Sun, M. S.; Liu, A. J.; Sun, Z. X.; Liu, T. T.; Cao, L.; Liang, L. J.; Qu, Q.; Li, Z. C.; Deng, Z. H.; Tong, Z. B.; Chu, G. C.; Tian, X. L.; Deng, H. T.; Zhao, S. W.; Li, J. B.; Lou, Z. Y.; Liu, L. H2B Lys34 Ubiquitination Induces Nucleosome Distortion to Stimulate Dot1L Activity. Nat. Chem. Biol. 2022, 18, 972–980; (b) Wang, Y.; Chen, J. N.; Hua, X.; Meng, X. B.; Cai, H. Y.; Wang, R. T.; Shi, J.; Deng, H. T.; Liu, L.; Li, Y. M. Photocaging of Activity-Based Ubiquitin Probes via a C-Terminal Backbone Modification Strategy. Angew. Chem. Int. Ed. 2022, 61, e202203792.
- 4(a) Balana, A. T.; Levine, P. M.; Craven, T. W.; Mukherjee, S.; Pedowitz, N. J.; Moon, S. P.; Takahashi, T. T.; Becker, C. F. W.; Baker, D.; Pratt, M. R. O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity. Nat. Chem. 2021, 13, 441–450; (b) Li, H. X.; Zhang, J.; An, C. J.; Dong, S. W. Probing N-Glycan Functions in Human Interleukin-17A Based on Chemically Synthesized Homogeneous Glycoforms. J. Am. Chem. Soc. 2021, 143, 2846–2856; (c) Ye, F. R.; Zhao, J.; Xu, P.; Liu, X. L.; Yu, J.; Shangguan, W. Z.; Liu, J. Z.; Luo, X. S.; Li, C.; Ying, T.; Wang, J.; Yu, B.; Wang, P. Synthetic Homogeneous Glycoforms of the SARS-CoV-2 Spike Receptor-Binding Domain Reveals Different Binding Profiles of Monoclonal Antibodies. Angew. Chem. Int. Ed. 2021, 60, 12904–12910; (d) Yang, W. Z.; Ramadan, S.; Orwenyo, J.; Kakeshpour, T.; Diaz, T.; Eken, Y.; Sanda, M.; Jackson, J. E.; Wilson, A. K.; Huang, X. F. Chemoenzymatic synthesis of glycopeptides bearing rare N-glycan sequences with or without bisecting GlcNAc. Chem. Sci. 2018, 9, 8194–8206; (e) Zhao, J.; Ye, F. R.; Huang, P.; Wang, P. Recent advances in chemical synthesis of O-linked glycopeptides and glycoproteins: An advanced synthetic tool for exploring the biological realm. Curr. Opin. Chem. Biol. 2023, 77, 102405; (f) Zhao, J.; Liu, X. L.; Liu, J. L.; Ye, F. R.; Wei, B. C.; Deng, M. G.; Li, T. H.; Huang, P.; Wang, P. Chemical synthesis creates single glycoforms of the ectodomain of herpes simplex virus-1 glycoprotein D. J. Am. Chem. Soc. 2023, https://doi.org/10.1021/jacs.3c11543.
- 5(a) Ai, H. S.; Chu, G. C.; Gong, Q. Y.; Tong, Z. B.; Deng, Z. H.; Liu, X.; Yang, F.; Xu, Z.; Li, J. B.; Tian, C. L.; Liu, L. Chemical Synthesis of Post-Translationally Modified H2AX Reveals Redundancy in Interplay between Histone Phosphorylation, Ubiquitination, and Methylation on the Binding of 53BP1 with Nucleosomes. J. Am. Chem. Soc. 2022, 144, 18329–18337; (b) Chu, N.; Salguero, A. L.; Liu, A. Z.; Chen, Z.; Dempsey, D. R.; Ficarro, S. B.; Alexander, W. M.; Marto, J. A.; Li, Y.; Amzel, L. M.; Gabelli, S. B.; Cole, P. A. Akt Kinase Activation Mechanisms Revealed Using Protein Semisynthesis. Cell 2018, 174, 897–907.e14; (c) Duan, H. Z.; Hu, C.; Li, Y. L.; Wang, S. H.; Xia, Y.; Liu, X.; Wang, J.; Chen, Y. X. Genetically Encoded Phosphine Ligand for Metalloprotein Design. J. Am. Chem. Soc. 2022, 144, 22831–22837; (d) Hauser, A.; Penkert, M.; Hackenberger, C. P. R. Chemical Approaches to Investigate Labile Peptide and Protein Phosphorylation. Acc. Chem. Res. 2017, 50, 1883–1893.
- 6(a) Tan, Y.; Wu, H. X.; Wei, T. Y.; Li, X. C. Chemical Protein Synthesis: Advances, Challenges, and Outlooks. J. Am. Chem. Soc. 2020, 142, 20288–20298; (b) Agouridas, V.; El Mahdi, O.; Melnyk, O. Chemical Protein Synthesis in Medicinal Chemistry. J. Med. Chem. 2020, 63, 15140–15152.
- 7(a) Pihl, R.; Zheng, Q. F.; David, Y. Nature-inspired protein ligation and its applications. Nat. Rev. Chem. 2023, 7, 234–255; (b) Thompson, R. E.; Muir, T. W. Chemoenzymatic Semisynthesis of Proteins. Chem. Rev. 2019, 120, 3051–3126; (c) Vamisetti, G. B.; Satish, G.; Sulkshane, P.; Mann, G.; Glickman, M. H.; Brik, A. On-Demand Detachment of Succinimides on Cysteine to Facilitate (Semi)Synthesis of Challenging Proteins. J. Am. Chem. Soc. 2020, 142, 19558–19569.
- 8 Roller, R. F.; Malik, A.; Carillo, M. A.; Garg, M.; Rella, A.; Raulf, M. K.; Lepenies, B.; Seeberger, P. H.; Varón Silva, D. Semisynthesis of Functional Glycosylphosphatidylinositol-Anchored Proteins. Angew. Chem. Int. Ed. 2020, 59, 12035–12040.
- 9(a) Adams, A. L.; Cowper, B.; Morgan, R. E.; Premdjee, B.; Caddick, S.; Macmillan, D. Cysteine Promoted C-Terminal Hydrazinolysis of Native Peptides and Proteins. Angew. Chem. Int. Ed. 2013, 52, 13062–13066; (b) Pan, M.; Zheng, Q. Y.; Ding, S.; Zhang, L. J.; Qu, Q.; Wang, T.; Hong, D. N.; Ren, Y. J.; Liang, L. J.; Chen, C. L.; Mei, Z. Q.; Liu, L. Chemical Protein Synthesis Enabled Mechanistic Studies on the Molecular Recognition of K27-linked Ubiquitin Chains. Angew. Chem. Int. Ed. 2019, 58, 2627–2631; (c) Pan, M.; Gao, S.; Zheng, Y.; Tan, X. D.; Lan, H.; Tan, X. L.; Sun, D. M.; Lu, L. N.; Wang, T.; Zheng, Q. Y.; Huang, Y. C.; Wang, J. W.; Liu, L. Quasi-Racemic X-ray Structures of K27-Linked Ubiquitin Chains Prepared by Total Chemical Synthesis. J. Am. Chem. Soc. 2016, 138, 7429.
- 10 Li, Q. Q.; Liu, Y. Q.; Luo, Y. Y.; Chu, T. T.; Gao, N.; Chen, P. G.; Chen, Y. X.; Li, Y. M. Uncovering the pathological functions of Ser404 phosphorylation by semisynthesis of a phosphorylated TDP-43 prion-like domain. Chem. Commun. 2020, 56, 5370–5373.
- 11(a) Mo, Z. Y.; Lin, S. M.; Chen, W. T.; He, C. M. Protein Ligation and Labeling Enabled by a C-Terminal Tetracysteine Tag. Angew. Chem. Int. Ed. 2022, 61, e202115377; (b) Qiao, Y. C.; Yu, G.; Kratch, K. C.; Wang, X. A.; Wang, W. W.; Leeuwon, S. Z.; Xu, S. Q.; Morse, J. S.; Liu, W. R. Expressed Protein Ligation without Intein. J. Am. Chem. Soc. 2020, 142, 7047–7054.
- 12 Yang, A.; Hacheney, I.; Wu, Y. W. Semisynthesis of autophagy protein LC3 conjugates. Med. Chem. 2017, 25, 4971–4976.
- 13 Ansaloni, A.; Wang, Z. M.; Jeong, J. S.; Ruggeri, F. S.; Dietler, G.; Lashuel, H. A. One-Pot Semisynthesis of Exon 1 of the Huntingtin Protein: New Tools for Elucidating the Role of Posttranslational Modifications in the Pathogenesis of Huntington's Disease. Angew. Chem. Int. Ed. 2014, 53, 1928–1933.
- 14 Margiola, S.; Gerecht, K.; Müller, M. M. Semisynthetic ‘designer’ p53 sheds light on a phosphorylation–acetylation relay. Chem. Sci. 2021, 12, 8563–8570.
- 15(a) Lin, S. M.; Mo, Z. Y.; Wang, P.; He, C. M. Oxidation and Phenolysis of Peptide/Protein C-Terminal Hydrazides Afford Salicylaldehyde Ester Surrogates for Chemical Protein Synthesis. J. Am. Chem. Soc. 2023, 145, 16843–16851; (b) Reif, A.; Siebenhaar, S.; Tröster, A.; Schmälzlein, M.; Lechner, C.; Velisetty, P.; Gottwald, K.; Pöhner, C.; Boos, I.; Schubert, V.; Rose-John, S.; Unverzagt, C. Semisynthesis of Biologically Active Glycoforms of the Human Cytokine Interleukin 6. Angew. Chem. Int. Ed. 2014, 53, 12125–12131; (c) Liao, P. S.; Liu, H. M.; He, C. M. Chemical synthesis of human selenoprotein F and elucidation of its thiol-disulfide oxidoreductase activity. Chem. Sci. 2022, 13, 6322–6327; (d) Li, Y. T.; Yi, C.; Chen, C. C.; Lan, H.; Pan, M.; Zhang, S. J.; Huang, Y. C.; Guan, C. J.; Li, Y. M.; Yu, L.; Liu, L. A semisynthetic Atg3 reveals that acetylation promotes Atg3 membrane binding and Atg8 lipidation. Nat. Commun. 2017, 8, 14848.
- 16 Hickey, C. M.; Wilson, N. R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell. Biol. 2012, 13, 755–766.
- 17(a) Dardashti, R. N.; Laps, S.; Gichtin, J. S.; Metanis, N. The semisynthesis of nucleolar human selenoprotein H. Chem. Sci. 2023, 14, 12723–12729. Metanis and co-workers reported the synthesis of selenoprotein H via SUMO fusion to increase thioester solubility. However, the critical thioester with internal Cys reported herein was not investigated; (b) Lamer, T.; van Belkum, M. J.; Wijewardane, A.; Chiorean, S.; Martin-Visscher, L. A.; Vederas, J. C. SPI “sandwich”: Combined SUMO-Peptide-Intein expression system and isolation procedure for improved stability and yield of peptides. Protein Sci. 2022, 31, e4316.
- 18 Wang, S.; Zhou, Q.; Li, Y.; Wei, B.; Liu, X.; Zhao, J.; Ye, F.; Zhou, Z.; Ding, B.; Wang, P. Quinoline-Based Photolabile Protection Strategy Facilitates Efficient Protein Assembly. J. Am. Chem. Soc. 2022, 144, 1232–1242.
- 19 Harmand, T. J.; Pattabiraman, V. R.; Bode, J. W. Chemical Synthesis of the Highly Hydrophobic Antiviral Membrane-Associated Protein IFITM3 and Modified Variants. Angew. Chem. Int. Ed. 2017, 56, 12639–12643.
- 20(a) Huang, D. L.; Li, Y.; Liang, J.; Yu, L.; Xue, M.; Cao, X. X.; Xiao, B.; Tian, C. L.; Liu, L.; Zheng, J. S. The New Salicylaldehyde S,S-Propanedithioacetal Ester Enables N-to-C Sequential Native Chemical Ligation and Ser/Thr Ligation for Chemical Protein Synthesis. J. Am. Chem. Soc. 2020, 142, 8790–8799; (b) Zheng, J. S.; Yu, M.; Qi, Y. K.; Tang, S.; Shen, F.; Wang, Z. P.; Xiao, L.; Zhang, L. H.; Tian, C. L.; Liu, L. J. Am. Chem. Soc. 2014, 136, 3695–3704.
- 21(a) Lenza, M. P.; Egia-Mendikute, L.; Antoñana-Vildosola, A.; Soares, C. O.; Coelho, H.; Corzana, F.; Bosch, A.; Manisha, P.; Quintana, J. I.; Oyenarte, I.; Unione, L.; Moure, M. J.; Azkargorta, M.; Atxabal, U.; Sobczak, K.; Elortza, F.; Sutherland, J. D.; Barrio, R.; Marcelo, F.; Jiménez-Barbero, J.; Palazon, A.; Ereño-Orbea, J. Structural insights into Siglec-15 reveal glycosylation dependency for its interaction with T cells through integrin CD11b. Nat. Commun. 2023, 14, 3496; (b) Wang, J.; Sun, J. W.; Liu, L. N.; Flies, D. B.; Nie, X. X.; Toki, M.; Zhang, J. P.; Song, C.; Zarr, M.; Zhou, X.; Han, X.; Archer, K. A.; O’Neill, T.; Herbst, R. S.; Boto, A. N.; Sanmamed, M. F.; Langermann, S.; Rimm, D. L.; Chen, L. P. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666.
- 22 Roberts, A. G.; Johnston, E. V.; Shieh, J.-H.; Sondey, J. P.; Hendrickson, R. C.; Moore, M. A. S.; Danishefsky, S. J. Fully Synthetic Granulocyte Colony-Stimulating Factor Enabled by Isonitrile-Mediated Coupling of Large, Side-Chain-Unprotected Peptides. J. Am. Chem. Soc. 2015, 137, 13167–13175.
- 23 Kerul, L.; Schrems, M.; Schmid, A.; Meli, R.; Becker, C. F. W.; Bello, C. Semisynthesis of Homogeneous, Active Granulocyte Colony Stimulating Factor Glycoforms. Angew. Chem. Int. Ed. 2022, 61, e202206116.
- 24 Zhao, J.; Liu, J. Z.; Liu, X. N.; Cao, Q.; Zhao, H. B.; Liu, L. Z.; Ye, F. R.; Wang, C.; Shao, H.; Xue, D. X.; Tao, H. C.; Li, B.; Yu, B.; Wang, P. Revealing Functional Significance of Interleukin-2 Glycoproteoforms Enabled by Expressed Serine Ligation. Chin. J. Chem. 2022, 40, 787–793.
- 25(a) Sun, Z. Q.; Ma, W. J.; Cao, Y. H.; Wei, T. Y.; Mo, X. Y.; Chow, H. Y.; Tan, Y.; Cheung, C. H. P.; Liu, J. M.; Lee, H. K.; Tse, E. C. M.; Liu, H.; Li, X. C. Superfast desulfurization for protein chemical synthesis and modification. Chem 2022, 8, 2542–2557; (b) Wan, Q.; Danishefsky, S. J. Free-Radical-Based, Specific Desulfurization of Cysteine: A Powerful Advance in the Synthesis of Polypeptides and Glycopolypeptides. Angew. Chem. Int. Ed. 2007, 46, 9248–9252.




