Visible-Light-Driven Four-Component Radical Relay Aminocarbonylation of Unactivated Alkenes†
Bin Lu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Shuo Bao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
These authors contributed equally to this work.
Search for more papers by this authorZi-Wei He
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Search for more papers by this authorWen-Jing Xiao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, 7 North Bingang Road, Wuhan, Hubei, 430083 China
Search for more papers by this authorCorresponding Author
Jia-Rong Chen
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, 7 North Bingang Road, Wuhan, Hubei, 430083 China
Key Laboratory of Organo-Pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, Ganzhou, Jiangxi, 341000 China
*E-mail: [email protected]Search for more papers by this authorBin Lu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Shuo Bao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
These authors contributed equally to this work.
Search for more papers by this authorZi-Wei He
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Search for more papers by this authorWen-Jing Xiao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, 7 North Bingang Road, Wuhan, Hubei, 430083 China
Search for more papers by this authorCorresponding Author
Jia-Rong Chen
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, 7 North Bingang Road, Wuhan, Hubei, 430083 China
Key Laboratory of Organo-Pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, Ganzhou, Jiangxi, 341000 China
*E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of C1 Chemistry.
Comprehensive Summary
Catalytic four-component radical carbonylation of unactivated alkenes has recently been recognized as a robust protocol for rapid construction of various structurally diverse carbonyl compounds. Given the significance of fluorine-containing groups, this reaction class has been extensively applied to assembly of a variety of perfluoroalkyl carboxylic acid derivatives by transition metal catalysis. Herein, we report a visible-light-driven radical relay 1,2-perfluoroalkylation aminocarbonylation of unactivated alkenes using CO gas as carbonyl source and 4CzIPN as organic photocatalyst. A wide range of alkenes and amines were well tolerated, providing the valuable β-perfluoroalkylated amides with generally good yields and high chemoselectivity.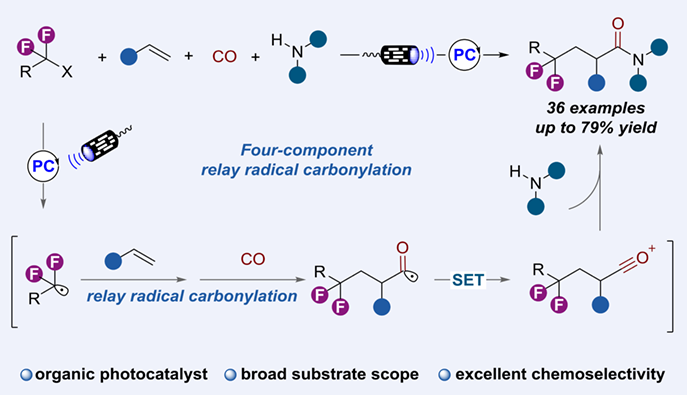
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300733-sup-0001-supinfo.pdfPDF document, 10.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected books and reviews, see: (a) Beller, M.; Wu, X.-F. Transition Metal Catalyzed Carbonylation Reactions; Springer: Heidelberg, 2013;
10.1007/978-3-642-39016-6 Google Scholar(b) Kollár, L. Modern Carbonylation Methods; Wiley-VCH, Weinheim, 2008;10.1002/9783527621545 Google Scholar(c) Brennfuhrer, A.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133; (d) Liu, Q.; Zhang, H.; Lei, A. Oxidative carbonylation reactions: organometallic compounds (R-M) or hydrocarbons (R-H) as nucleophiles. Angew. Chem. Int. Ed. 2011, 50, 10788–10799; (e) Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013, 113, 1–35; (f) Wu, X. F.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account. Acc. Chem. Res. 2014, 47, 1041–1153; (g) Li, Y.; Hu, Y.; Wu, X. F. Non-noble metal-catalysed carbonylative transformations. Chem. Soc. Rev. 2018, 47, 172–194; (h) Peng, J.-B.; Wu, F.-P.; Wu, X.-F. First-Row Transition-Metal-Catalyzed Carbonylative Transfor-mations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127; (i) Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526–552; (j) Zhang, S.; Neumann, H.; Beller, M. Synthesis of α,β-unsaturated carbonyl compounds by carbonylation reactions. Chem. Soc. Rev. 2020, 49, 3187–3210; (k) Zhu, C.; Liu, J.; Li, M. B.; Backvall, J. E., Palladium-catalyzed oxidative dehydrogenative carbonylation reactions using carbon monoxide and mechanistic overviews. Chem. Soc. Rev. 2020, 49, 341–353; (l) Cheng, L. J.; Mankad, N. P. Copper-Catalyzed Carbonylative Coupling of Alkyl Halides. Acc. Chem. Res. 2021, 54, 2261–2274; (m) Liu, Y.; Chen, Y.-H.; Yi, H.; Lei, A. An Update on Oxidative C–H Carbonylation with CO. ACS Catal. 2022, 12, 7470–7485; (n) Li, W.; Jiang, D.; Wang, C.; Cheng, L.-J. Recent Advances in Base-Metal-Catalyzed Carbonylation of Unactivated Alkyl Electrophiles. Chin. J. Chem. 2023. 41, 3419–3432; (o) Chen, X.; Chen, G.; Lian, Z. Recent Advances in Nickel Catalyzed Carbonylative Reactions via the Insertion of Carbon Monoxide. Chin. J. Chem. 2023, 42, 177–189.
- 2For selected reviews and recent works, see: (a) Shen, C.; Wu, X.-F. Palladium-Catalyzed Carbonylative Multicomponent Reactions. Chem. Eur. J. 2017, 23, 2973–2987; (b) Peng, J.-B. Recent Advances in Carbonylative Difunctionalization of Alkenes. Adv. Synth. Catal. 2020, 362, 3059–3080; (c) Xu, J.-X.; Wang, L.-C.; Wu, X.-F. Non-Noble Metal-Catalyzed Carbonylative Multi-Component Reactions. Chem. Asian J. 2022, 17, e202200928; (d) Kuai, C.-S.; Teng, B.-H.; Wu, X.-F. Palladium-Catalyzed Carbonylative Multicomponent Fluoroalkylation of 1,3-Enynes: Concise Construction of Diverse Cyclic Compounds. Angew. Chem. Int. Ed. 2023, 62, e202318257.
- 3(a) Tsuji, J.; Morikawa, M.; Kiji, J. Organic syntheses by means of noble metal compounds X. Carbonylation reaction of cyclopropane catalyzed by palladium chloride. Tetrahedron Lett. 1965, 6, 817–819;
10.1016/S0040-4039(00)90024-8 Google Scholar(b) Tsuji, J.; Iwamoto, N. Organic synthesis by means of noble-metal compounds. Palladium-catalyzed carbonylation of amines. Chem. Commun. 1966, 380.
- 4(a) Schoenberg, A.; Bartoletti, I.; Heck, R. F. Palladium-catalyzed carboalkoxylation of aryl, benzyl, and vinylic halides. J. Org. Chem. 1974, 39, 3318–3326; (b) Schoenberg, A.; Heck, R. F. Palladium-catalyzed formylation of aryl, heterocyclic, and vinylic halides. J. Am. Chem. Soc. 1974, 96, 7761–7764; (c) Schoenberg, A.; Heck, R. F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J. Org. Chem. 1974, 39, 3327–3331.
- 5For selected reviews on radical carbonylation chemistry, see: (a) Ryu, I.; Sonoda, N. Free-Radical Carbonylations: Then and Now. Angew. Chem. Int. Ed. 1996, 35, 1050–1066; (b) Ryu, I. Radical carboxylations of iodoalkanes and saturated alcohols using carbon monoxide. Chem. Soc. Rev. 2001, 30, 16–25; (c) Schiesser, C. H.; Wille, U.; Matsubara, H.; Ryu, I. Radicals masquerading as electrophiles: dual orbital effects in nitrogen-philic acyl radical cyclization and related addition reactions. Acc. Chem. Res. 2007, 40, 303–313; (d) Matsubara, H.; Kawamoto, T.; Fukuyama, T.; Ryu, I. Applications of Radical Carbonylation and Amine Addition Chemistry: 1,4-Hydrogen Transfer of 1-Hydroxylallyl Radicals. Acc. Chem. Res. 2018, 51, 2023–2035; (e) Zhao, S.; Mankad, N. P. Metal-catalysed radical carbonylation reactions. Catal. Sci. Technol. 2019, 9, 3603–3613.
- 6(a) Brubaker, M. M.; Coffman, D. D.; Hoehn, H. H. Synthesis and Characterization of Ethylene/Carbon Monoxide Copolymers, a New Class of Polyketones1. J. Am. Chem. Soc. 1952, 74, 1509–1515; (b) Coffman, D. D.; Pinkney, P. S.; Wall, F. T.; Wood, W. H.; Young, H. S. Compositional Relationships in the Copolymerization of Ethylene with Carbon Monoxide. J. Am. Chem. Soc. 1952, 74, 3391–3393; (c) Foster, R. E.; Larchar, A. W.; Lipscomb, R. D.; McKusick, B. C., Telomers from Carbon Monoxide and Olefins. J. Am. Chem. Soc. 1956, 78, 5606–5611; (d) Sauer, J. C. Free Radical-initiated Reactions of Acetylene with Thiols and Carbon Monoxide. J. Am. Chem. Soc. 1957, 79, 5314–5315.
- 7(a) Ryu, I.; Yamazaki, H.; Ogawa, A.; Kambe, N.; Sonoda, N. Four carbon component coupling reaction based on free-radical carbonylation: an easy access to β-functionalized δ,ε-unsaturated ketones. J. Am. Chem. Soc. 1993, 115, 1187–1189; (b) Miura, K.; Tojino, M.; Fujisawa, N.; Hosomi, A.; Ryu, I. Cascade Carbonylation Methods Leading to β-Diketones and β-Functionalized δ-Diketones. Angew. Chem. Int. Ed. 2004, 43, 2423–2425.
- 8(a) Julia, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352; (b) Chen, J.-J.; Huang, H.-M. Merging Halogen-Atom transfer with transition metal catalysis. Tetrahedron Lett. 2022, 102, 153945.
- 9 Fusano, A.; Sumino, S.; Nishitani, S.; Inouye, T.; Morimoto, K.; Fukuyama, T.; Ryu, I. Pd/light-accelerated atom-transfer carbonylation of alkyl iodides: applications in multicomponent coupling processes leading to functionalized carboxylic acid derivatives. Chem. Eur. J. 2012, 18, 9415–9422.
- 10(a) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Carbonylation reactions of alkyl iodides through the interplay of carbon radicals and Pd catalysts. Acc. Chem. Res. 2014, 47, 1563–1574; (b) Liu, Q.; Dong, X.; Li, J.; Xiao, J.; Dong, Y.; Liu, H. Recent Advances on Palladium Radical Involved Reactions. ACS Catal. 2015, 5, 6111–6137.
- 11 Zhang, Y.; Geng, H. Q.; Wu, X.-F. Palladium-Catalyzed Perfluoroalkylative Carbonylation of Unactivated Alkenes: Access to beta-Perfluoroalkyl Esters. Angew. Chem. Int. Ed. 2021, 60, 24292–24298.
- 12 Bao, Z.-P.; Zhang, Y.; Wu, X.-F. Palladium-catalyzed four-component difluoroalkylative carbonylation of aryl olefins and ethylene. J. Catal. 2022, 413, 163–167.
- 13 Wu, F.-P.; Yuan, Y.; Wu, X.-F. Copper-Catalyzed 1,2-Trifluoromethylation Carbonylation of Unactivated Alkenes: Efficient Access to beta-Trifluoromethylated Aliphatic Carboxylic Acid Derivatives. Angew. Chem. Int. Ed. 2021, 60, 25787–25792.
- 14 Zhang, Y.; Yuan, Y.; Geng, H.-Q.; Xu, J.-X.; Wu, X.-F. Visible light-induced perfluoroalkylative carbonylation of unactivated alkenes. J. Catal. 2022, 413, 214–220.
- 15 Wang, Y.; Wang, P.; Neumann, H.; Beller, M., Cobalt-Catalyzed Multicomponent Carbonylation of Olefins: Efficient Synthesis of β-Perfluoroalkyl Imides, Amides, and Esters. ACS Catal. 2023, 13, 6744–6753.
- 16 Lu, B.; Zhang, Z.; Jiang, M.; Liang, D.; He, Z.-W.; Bao, F.-S.; Xiao, W.-J.; Chen, J.-R. Photoinduced Five-Component Radical Relay Aminocarbonylation of Alkenes. Angew. Chem. Int. Ed. 2023, 62, e202309460.
- 17(a) Lima, F.; Sharma, U. K.; Grunenberg, L.; Saha, D.; Johannsen, S.; Sedelmeier, J.; Van der Eycken, E. V.; Ley, S. V. A Lewis Base Catalysis Approach for the Photoredox Activation of Boronic Acids and Esters. Angew. Chem. Int. Ed. 2017, 56, 15136–15140; (b) Lima, F.; Grunenberg, L.; Rahman, H. B. A.; Labes, R.; Sedelmeier, J.; Ley, S. V. Organic photocatalysis for the radical couplings of boronic acid derivatives in batch and flow. Chem. Commun. 2018, 54, 5606–5609; (c) Sun, X.; Chapin, B. M.; Metola, P.; Collins, B.; Wang, B.; James, T. D.; Anslyn, E. V. The mechanisms of boronate ester formation and fluorescent turn-on in ortho-aminomethylphenylboronic acids. Nat. Chem. 2019, 11, 768–778; (d) Gockel, S. N.; Lee, S.; Gay, B. L.; Hull, K. L. Oxidative Three-Component Carboamination of Vinylarenes with Alkylboronic Acids. ACS Catal. 2021, 11, 5166–5171.
- 18 Ruos, M. E.; Kinney, R. G.; Ring, O. T.; Doyle, A. G. A General Photocatalytic Strategy for Nucleophilic Amination of Primary and Secondary Benzylic C-H Bonds. J. Am. Chem. Soc. 2023, 145, 18487–18496.




