Synthesis, Structure and Chemical Bonding of Polyantimony Clusters Containing Coinage Metals [M2Sb14]4– (M = Cu, Ag)
Wei-Xing Chen
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorYu-He Xu
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorCui-Cui Wang
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorLei Qiao
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorZi-Yan Guo
Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi, 030006 China
Search for more papers by this authorCorresponding Author
Wen-Juan Tian
Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi, 030006 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhong-Ming Sun
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorWei-Xing Chen
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorYu-He Xu
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorCui-Cui Wang
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorLei Qiao
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorZi-Yan Guo
Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi, 030006 China
Search for more papers by this authorCorresponding Author
Wen-Juan Tian
Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi, 030006 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhong-Ming Sun
State Key Laboratory of Element-Organic Chemistry, Tianjin Key Lab for Rare Earth Materials and Applications, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorComprehensive Summary
Binary polyantimony clusters, namely [Cu2Sb14]4– and [Ag2Sb14]4–, containing coinage metals, were successfully synthesized and characterized, in which two homoatomic Sb73– subunits are bridged by two coinage metals in η4:η1 and η1:η1 coordination modes, respectively. In [Cu2Sb14]4–, two bridging Cu ions and two Sb atoms form a planar rhombic unit, which was revealed to have antiaromatic properties by theoretical calculations. Further electron structure and bonding analysis confirmed the presence of delocalized bonds in the rhombic unit and the metallophilic interaction between two formal M+ ions.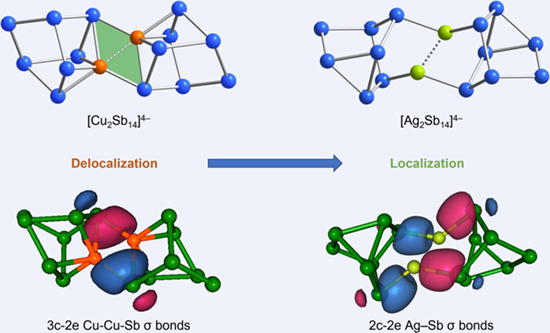
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300606-sup-0001-supinfo.pdfPDF document, 1.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Wilson, R. J.; Lichtenberger, N.; Weinert, B.; Dehnen, S. Intermetalloid and Heterometallic Clusters Combining p-Block (Semi)Metals with d- or f-Block Metals. Chem. Rev. 2019, 119, 8506–8554.
- 2 Turbervill, R.; Goicoechea, J. M. From clusters to unorthodox pnictogen sources: solution-phase reactivity of [E7]3- (E = P-Sb) anions. Chem. Rev. 2014, 114, 10807–10828.
- 3 Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T. F. Zintl ions, cage compounds, and intermetalloid clusters of Group 14 and Group 15 elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670.
- 4 Perla, L. G.; Oliver, A. G.; Sevov, S. C. Bi73-: The missing family member, finally isolated and characterized. Inorg. Chem. 2015, 54, 872–875.
- 5 Weinert, B.; Eulenstein, A. R.; Ababei, R.; Dehnen, S. Formation of [Bi11]3−, a homoatomic, polycyclic bismuth polyanion, by pyridine-assisted decomposition of [GaBi3]2−. Angew. Chem. Int. Ed. 2014, 53, 4704–4708.
- 6 Charles, S.; Eichhorn, B. W.; Rheingold, A. L.; Bott, S. G. Synthesis, Structure, and Properties of the [E7M(CO)3]3− Complexes Where E = P, As, Sb and M = Cr, Mo, W. J. Am. Chem. Soc. 1994, 116, 8077–8086.
- 7 Eichhorn, B. W.; Haushalter, R. C.; Huffman, J. C. Insertion of Cr(CO)3 into As to Form [As7Cr(CO)3]3−: An Inorganic Nortricyclane-to-Norbornadiene Conversion. Angew. Chem. Int. Ed. 1989, 28, 1032–1033.
- 8
Haushalter, R. C.; Eichhorn, B. W.; Rheingold, A. L.; Geib, S. J. Oxidatively coupled polyarsenide clusters: synthesis and structures of SnAs144– and As224–. J. Chem. Soc., Chem. Commun. 1988, 1027–1028.
10.1039/c39880001027 Google Scholar
- 9 Mandal, S.; Reber, A. C.; Qian, M., Liu, R.; Saavedra, H. M.; Sen, S.; Weiss, P. S.; Khanna, S. N.; Sen, A. Synthesis, structure and band gap energy of covalently linked cluster-assembled materials. Dalton Trans. 2012, 41, 12365–12377.
- 10 Kaas, M.; Korber, N. Synthesis and characterization of [Rb([18]crown-6)]3As15Zn·4NH3 and [Rb([2.2.2]crypt)]4Sb14Zn·8NH3. Z. Anorg. Allg. Chem. 2017, 643, 1331–1334.
- 11 Knapp, C.; Zhou, B.; Denning, M. S.; Rees, N. H.; Goicoechea, J. M. Reactivity studies of group 15 Zintl ions towards homoleptic post-transition metal organometallics: a ‘bottom-up’ approach to bimetallic molecular clusters. Dalton Trans. 2010, 39, 426–436.
- 12 Lichtenberger, N.; Massa, W.; Dehnen, S. Polybismuthide anions as ligands: the homoleptic complex [(Bi7)Cd(Bi7)]4− and the ternary cluster [(Bi6)Zn3(TlBi5)]4−. Angew. Chem. Int. Ed. 2019, 58, 3222–3226.
- 13 Knapp, C. M.; Large, J. S.; Rees, N. H.; Goicoechea, J. M. A versatile salt-metathesis route to heteroatomic clusters derived from phosphorus and arsenic Zintl anion. Dalton Trans. 2011, 40, 735–745.
- 14 Zhang, W. Q.; Tkachenko, N. V.; Qiao, L.; Boldyrev, A. I.; Sun, Z. M. Synthesis and structure of binary copper/silver–arsenic clusters derived from Zintl ion As73–. Chin. J. Chem. 2022, 40, 65–70.
- 15 Chaki, N. K.; Mandal, S.; Reber, A. C.; Qian, M.; Saavedra, H. M.; Weiss, P. S.; Khanna, S. N.; Sen, A. Controlling band gap energies in cluster-assembled ionic solids through internal electric fields. ACS Nano 2010, 4, 5813–5818.
- 16 Moses, M. J.; Fettinger, J.; Eichhorn, B. W. Charged molecular alloys: synthesis and characterization of the binary anions Pd7As164- and Pd2As144-. J. Am. Chem. Soc. 2002, 124, 5944–5944.
- 17 Knapp, C. M.; Jackson, C. S.; Large, J. S.; Thompson, A. L.; Goicoechea, J. M. Heteroatomic molecular clusters derived from group 15 Zintl ion cages: synthesis and isolation of [M2(HP7)2]2− (M = Ag, Au), two novel cluster anions exhibiting metallophilic interactions. Inorg. Chem. 2011, 50, 4021–4028.
- 18 Charles, S.; Eichhorn, B. W.; Bott, S. G. Synthesis and structure of [Sb7Ni3(CO)3]3-: a new structural type for nido 10-vertex polyhedral clusters. J. Am. Chem. Soc. 1993, 115, 5837–5838.
- 19 Pan, F. X.; Li, L. J.; Wang, Y. J.; Guo, J. C.; Zhai, H. J.; Xu, L.; Sun, Z. M. An All-Metal Aromatic Sandwich Complex [Sb3Au3Sb3]3-. J. Am. Chem. Soc. 2015, 137, 10954–10957.
- 20 Kesanli, B.; Fettinger, J.; Eichhorn, B. Controlled aggregation of ME8n- binary anions (M = Cr, Mo; E = As, Sb) into one-dimensional arrays: Structures, magnetism and spectroscopy. J. Am. Chem. Soc. 2003, 125, 7367–7376.
- 21 Wang, Y.; Zavalij, P.; Eichhorn, B. [(ZnSb6)2]4-: a new structure type for coupled norbornadiene-like subunits. Chem. Commun. 2017, 53, 11600–11602.
- 22 Liu, C.; Tkachenko, N. V.; Popov, I. A.; Fedik, N.; Min, X.; Xu, C. Q.; Li, J.; McGrady, J. E.; Boldyrev, A. I.; Sun, Z. M. Structure and Bonding in [Sb@In8Sb12]3- and [Sb@In8Sb12]5-. Angew. Chem. Int. Ed. 2019, 58, 8367–8371.
- 23 Moses, M. J.; Fettinger, J. C.; Eichhorn, B. W. [Ni5Sb17]4- transition-metal Zintl ion complex: crossing the Zintl border in molecular intermetalloid clusters. Inorg. Chem. 2007, 46, 1036–1038.
- 24 Min, X.; Popov, I. A.; Pan, F. X.; Li, L. J.; Matito, E.; Sun, Z. M.; Wang, L. S.; Boldyrev, A. I. All-Metal Antiaromaticity in Sb4-Type Lanthanocene Anions. Angew. Chem. Int. Ed. 2016, 55, 5531–5535.
- 25 Popov, I. A.; Pan, F. X.; You, X. R.; Li, L. J.; Matito, E.; Liu, C.; Zhai, H. J.; Sun, Z. M.; Boldyrev, A. I. Peculiar All-Metal σ-Aromaticity of the [Au2Sb16]4- Anion in the Solid State. Angew. Chem. Int. Ed. 2016, 55, 15344–15346.
- 26 Wang, Y.; Zavalij, P.; Eichhorn, B. The cyclo-Sb6 ring in the [Sb6(RuCp*)2]2- ion. Chem. Commun. 2018, 54, 11917–11920.
- 27 Ruan, H.; Wang, L.; Li, Z.; Xu, L. Sb102- and Sb22- found in [K(18-crown-6)]6[Sb10][Sb4{Mo(CO)3}2]∙2en: two missing family members. Dalton Trans. 2017, 46, 7219–7222.
- 28 Lin, L.; Chen, S.; Lu, Z.; Xu, L. Unprecedented icosahedral clusters built of polyantimony: from single [Ni0.5@{Sb6Ni6(CO)8}]4- and [Ni@{Sb7Ni5(CO)6}]3- to the Sb84--linked dimer [(Sb8){Sb7Ni5(CO)4}2]6-. Inorg. Chem. Front. 2021, 8, 5086–5092.
- 29 Wang, Y.; Moses-DeBusk, M.; Stevens, L.; Hu, J.; Zavalij, P.; Bowen, K.; Dunlap, B. I.; Glaser, E. R.; Eichhorn, B. Sb@Ni12@Sb20-/+ and Sb@Pd12@Sb20n Cluster Anions, Where n = +1, -1, -3, -4: Multi-Oxidation-State Clusters of Interpenetrating Platonic Solids. J. Am. Chem. Soc. 2017, 139, 619–622.
- 30 Li, Z.; Ruan, H.; Wang, L.; Liu, C.; Xu, L. Counterion-induced crystallization of intermetalloid Matryoshka clusters [Sb@Pd12@Sb20]3-,4-. Dalton Trans. 2017, 46, 3453–3456.
- 31
Yue, X.-H.; Chen, W.-X.; Yang, T.; Muñoz-Castro, A.; Frenking, G.; Sun, Z.-M. Carbon-free sandwich compounds based on arsenic and antimony with icosahedral metal cores. Nat. Synth. 2023, 2, 423–429.
10.1038/s44160-023-00247-0 Google Scholar
- 32 Moses, M. J.; Fettinger, J. C.; Eichhorn, B. W. Interpenetrating As20 fullerene and Ni12 icosahedra in the onion-skin [As@Ni12@As20]3- ion. Science 2003, 300, 778–780.
- 33 Wang, Z. C.; Tkachenko, N. V.; Qiao, L.; Matito, E.; Munoz-Castro, A.; Boldyrev, A. I.; Sun, Z. M. All-metal sigma-antiaromaticity in dimeric cluster anion {[CuGe9Mes]2}4-. Chem. Commun. 2020, 56, 6583–6586.
- 34 Wilson, R. J.; Broeckaert, L.; Spitzer, F.; Weigend, F.; Dehnen, S. {[CuSn5Sb3]2-}2: A Dimer of Inhomogeneous Superatoms. Angew. Chem. Int. Ed. 2016, 55, 11775–11780.
- 35 Xu, Y. H.; Tkachenko, N. V.; Popov, I. A.; Qiao, L.; Munoz-Castro, A.; Boldyrev, A. I.; Sun, Z. M. Ternary aromatic and anti-aromatic clusters derived from the hypho species [Sn2Sb5]3- Nat. Commun. 2021, 12, 4465.
- 36 Goodwin, A. L.; Calleja, M.; Conterio, M. J.; Dove, M. T.; Evans, J. S. O.; Keen, D. A.; Peters, L.; Tucker, M. G. Colossal Positive and Negative Thermal Expansion in the Framework Material Ag3[Co(CN)6]. Science 2008, 319, 794–797.
- 37 Liu, Z.; Yang, J.; Yang, L.; Li, X.; Ma, R.; Wang, R.; Xing, X.; Sun, D. Argentophilicity induced anomalous thermal expansion behavior in a 2D silver squarate. Inorg. Chem. Front. 2021, 8, 1567–1573.
- 38 Qiao, L.; Zhang, C.; Shu, C. C.; Morgan, H. W. T.; McGrady, J. E.; Sun, Z. M. [Cu4@E18]4- (E = Sn, Pb): Fused Derivatives of Endohedral Stannaspherene and Plumbaspherene. J. Am. Chem. Soc. 2020, 142, 13288–13293.
- 39 Charles, S.; Eichhorn, B. W.; Bott, S. G. Synthesis and structure of [Sb7Ni3(CO)3]3-: a new structural type for nido 10-vertex polyhedral clusters. J. Am. Chem. Soc. 1993, 115, 5837–5838.
- 40 Charles, S.; Eichhorn, B. W.; Rheingold, A. L.; Bott, S. G. Synthesis, Structure, and Properties of the [E7M(CO)3]3- Complexes Where E = P, As, Sb and M = Cr, Mo, W. J. Am. Chem. Soc. 1994, 116, 8077–8086.
- 41 Meyer, E. M.; Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastin, C. Polynuclear Aryl Derivatives of Group 11 Metals. Synthesis, Solid State-solution Structural Relationship, and Reactivity with Phosphines. Organometallics 1989, 8, 1067–1079.




