Palladium-Catalyzed anti-Markovnikov Halosulfonamidation of Unactivated Alkene†
Bowen Wang
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorJianxiao Li
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorWanqing Wu
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorCorresponding Author
Huanfeng Jiang
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
E-mail: [email protected]Search for more papers by this authorBowen Wang
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorJianxiao Li
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorWanqing Wu
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
Search for more papers by this authorCorresponding Author
Huanfeng Jiang
State Key Laboratory of Pulp and Paper Engineering, Key Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510641 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Palladium-catalyzed regioselective amination of unactivated alkene remains a challenge and is of great interest. Herein, a three-component coupling of unactivated alkene, sulfonamide, and N-halo compounds accessing vicinal haloamine has been conceived. This aminohalogenation represents a modular and regioselective strategy. The electrophilic halogenating agent enables regioselective anti-Markovnikov aminopalladation and facilitates subsequent halogenation events. And this protocol is characterized by gram-scale syntheses and late-stage functionalizations. Of note, the recovered byproduct phthalimide allows for reusing by conversion to the starting material.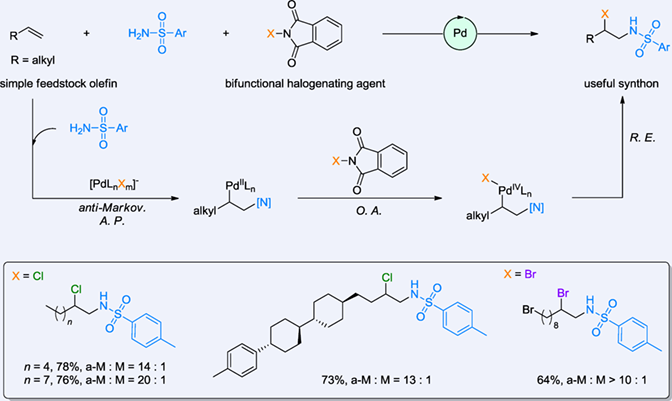
Supporting Information
| Filename | Description |
|---|---|
| cjoc2023000601-sup-0001-supinfo.pdfPDF document, 13.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Michael, F. E.; Sibbald, P. A.; Cochran, B. M. Palladium-Catalyzed Intramolecular Chloroamination of Alkenes. Org. Lett. 2008, 10, 793–796.
- 2 Wu, T.; Yin, G. Y.; Liu, G. S. Palladium-Catalyzed Intramolecular Aminofluorination of Unactivated Alkenes. J. Am. Chem. Soc. 2009, 131, 16354–16355.
- 3 White, P. B.; Stahl, S. S. Reversible Alkene Insertion into the Pd-N Bond of Pd(II)-Sulfonamidates and Implications for Catalytic Amidation Reactions. J. Am. Chem. Soc. 2011, 133, 18594–18597.
- 4 Yin, G. Y.; Wu, T.; Liu, G. S. Highly Selective Palladium-Catalyzed Intramolecular Chloroamination of Unactivated Alkenes by Using Hydrogen Peroxide as an Oxidant. Chem. Eur. J. 2012, 18, 451–455.
- 5 Chen, C. H.; Hou, C. Q.; Chen, P. H.; Liu, G. S. Palladium(II)-Catalyzed Aminotrifluoromethoxylation of Alkenes: Mechanistic Insight into the Effect of N-Protecting Groups. Chin. J. Chem. 2020, 38, 346–350.
- 6 Shi, L. J.; Wen, M. S.; Li, F. W. Palladium-Catalyzed Tandem Carbonylative Aza-Wacker-Type Cyclization of Nucleophile Tethered Alkene to Access Fused N-Heterocycles. Chin. J. Chem. 2021, 39, 317–322.
- 7 Liu, C.; Tan, X.; Zhan, L.; Jing, Y.; Wu, W.; Ke, Z.; Jiang, H. Palladium-Catalyzed Cascade Cyclization for the Synthesis of Fused Benzo-Aza-Oxa-[5-6-5] Tetracycles. Angew. Chem. Int. Ed. 2022, 61, e202215020.
- 8 Scott, K. A.; Njardarson, J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top Curr. Chem. 2018, 376, 5.
- 9 Supuran, C. T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249.
- 10 Brown-Elliott, B. A.; Nash, K. A.; Wallace, R. J. Antimicrobial Susceptibility Testing, Drug Resistance Mechanisms, and Therapy of Infections with Nontuberculous Mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582.
- 11 Li, G. G.; Kotti, S. R. S. S.; Timmons, C. Recent Development of Regio- and Stereoselective Aminohalogenation Reaction of Alkenes. Eur. J. Org. Chem. 2007, 2745–2758.
- 12 Alix, A.; Lalli, C.; Retailleau, P.; Masson, G. Highly Enantioselective Electrophilic α-Bromination of Enecarbamates: Chiral Phosphoric Acid and Calcium Phosphate Salt Catalysts. J. Am. Chem. Soc. 2012, 134, 10389–10392.
- 13 Bovino, M. T.; Chemler, S. R. Catalytic Enantioselective Alkene Aminohalogenation/Cyclization Involving Atom Transfer. Angew. Chem. Int. Ed. 2012, 51, 3923–3927.
- 14 Chemler, S. R.; Bovino, M. T. Catalytic Aminohalogenation of Alkenes and Alkynes. ACS Catal. 2013, 3, 1076–1091.
- 15 Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. Catalytic Diastereo- and Enantioselective Fluoroamination of Alkenes. J. Am. Chem. Soc. 2018, 140, 4797–4802.
- 16 Li, Y.; Liang, Y. J.; Deng, J. C.; Dong, Y.; Zhao, C. Y.; Su, Z. M.; Guan, W.; Bi, X. H.; Liu, Q.; Fu, J. K. Directed Copper-Catalyzed Intermolecular Aminative Difunctionalization of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 18475–18485.
- 17 Govaerts, S.; Angelini, L.; Hampton, C.; Malet-Sanz, L.; Ruffoni, A.; Leonori, D. Photoinduced Olefin Diamination with Alkylamines. Angew. Chem. Int. Ed. 2020, 59, 15021–15028.
- 18 Chen, X.; Xiao, F.; He, W. M. Recent developments in the difunctionalization of alkenes with C–N bond formation. Org. Chem. Front. 2021, 8, 5206–5228.
- 19 Xu, T.; Qiu, S. F.; Liu, G. S. Palladium-Catalyzed Intramolecular Aminofluorination of Styrenes. Chin. J. Chem. 2011, 29, 2785–2790.
- 20 Peng, H. H.; Yuan, Z. L.; Chen, P. H.; Liu, G. S. Palladium-Catalyzed Intermolecular Oxidative Diazidation of Alkenes. Chin. J. Chem. 2017, 35, 876–880.
- 21 Legnani, L.; Prina-Cerai, G.; Delcaillau, T.; Willems, S.; Morandi, B. Efficient access to unprotected primary amines by iron-catalyzed aminochlorination of alkenes. Science 2018, 362, 434–439.
- 22 Falk, E.; Makai, S.; Delcaillau, T.; Gurtler, L.; Morandi, B. Design and Scalable Synthesis of N-Alkylhydroxylamine Reagents for the Direct Iron-Catalyzed Installation of Medicinally Relevant Amines. Angew. Chem. Int. Ed. 2020, 59, 21064–21071.
- 23 Zhao, J.; Huang, H.-G.; Li, W.; Liu, W.-B. FeCl2-Mediated Regioselective Aminochlorination and Aminoazidation of Styrenes with Trifluoromethanesulfonyl Azide. Org. Lett. 2021, 23, 5102–5106.
- 24 Ruiz-Castillo, P.; Buchwald, P. S. L. Applications of Palladium-Catalyzed C−N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649.
- 25 Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E. Guiry, P. J.; Moberg, C.; Backväll, J. E.; Pfaltz, A.; Pericàs, M. A.; Diéguez, M. Recent Advances in Enantioselective Pd-Catalyzed Allylic Substitution: From Design to Applications. Chem. Rev. 2021, 121, 4373–4505.
- 26 Reichert, E. C.; Feng, K.; Sather, A. C.; Buchwald, S. L. Pd-Catalyzed Amination of Base-Sensitive Five-Membered Heteroaryl Halides with Aliphatic Amines. J. Am. Chem. Soc. 2023, 145, 3323–3329.
- 27 Muñiz, K. High-Oxidation-State Palladium Catalysis: New Reactivity for Organic Synthesis. Angew. Chem. Int. Ed. 2009, 48, 9412–9423.
- 28 Mann, S. E.; Benhamou, L.; Sheppard, T. D. Palladium(II)-Catalysed Oxidation of Alkenes. Synthesis 2015, 47, 3079–3117.
- 29 Weiner, B.; Baeza, A.; Jerphagnon, T.; Feringa, B. L. Aldehyde Selective Wacker Oxidations of Phthalimide Protected Allylic Amines: A New Catalytic Route to β3-Amino Acids. J. Am. Chem. Soc. 2009, 131, 9473–9474.
- 30 Bronner, S. M.; Grubbs, R. H. Formal anti-Markovnikov hydroamination of terminal olefins. Chem. Sci. 2014, 5, 101–106.
- 31 Dong, J. J.; Browne, W. R.; Feringa, B. L. Palladium-Catalyzed anti-Markovnikov Oxidation of Terminal Alkenes. Angew. Chem. Int. Ed. 2015, 54, 734–744.
- 32 Buchanan, T. L.; Hull, K. L. Illuminating Amination. Science 2017, 355, 690–691.
- 33 Hosokawa, T.; Takano, M.; Kuroki, Y.; Murahashi, S. I. Palladium(II)-Catalyzed Amidation of Alkenes. Tetrahedron Lett. 1992, 33, 6643–6646.
- 34 Timokhin, V. I.; Stahl, S. S. Brønsted Base-Modulated Regioselectivity in the Aerobic Oxidative Amination of Styrene Catalyzed by Palladium. J. Am. Chem. Soc. 2005, 127, 17888–17893.
- 35 Lee, J. M.; Ahn, D. S.; Jung, D. Y.; Lee, J.; Do, Y.; Kim, S. K.; Chang, S. K. Hydrogen-Bond-Directed Highly Stereoselective Synthesis of Z-Enamides via Pd-Catalyzed Oxidative Amidation of Conjugated Olefins. J. Am. Chem. Soc. 2006, 128, 12954–12962.
- 36 Ji, X.; Huang, H.; Wu, W.; Jiang, H. Palladium-Catalyzed Intermolecular Dehydrogenative Aminohalogenation of Alkenes under Molecular Oxygen: An Approach to Brominated Enamines. J. Am. Chem. Soc. 2013, 135, 5286–5289.
- 37 Ouyang, L.; Li, J.; Zheng, J.; Huang, J.; Qi, C.; Wu, W.; Jiang, H. Access to α-Amino Acid Esters through Palladium-Catalyzed Oxidative Amination of Vinyl Ethers with Hydrogen Peroxide as the Oxidant and Oxygen Source. Angew. Chem. Int. Ed. 2017, 56, 15926–15930.
- 38Wu; Z.; Hu, M.; Jin, Y.; Li, J.; Wu, W.; Jiang, H. Synthesis of medicinally relevant oxalylamines via copper/Lewis acid synergistic catalysis. Sci. Adv. 2021, 7, eabh4088.
- 39 Gurak, J. A.; Yang, K. S.; Liu, Z.; Engle, K. M. Directed, Regiocontrolled Hydroamination of Unactivated Alkenes via Protodepalladation. J. Am. Chem. Soc. 2016, 138, 5805–5808.
- 40 Liu, Z.; Wang, Y. Y.; Wang, Z. C.; Zeng, T.; Liu, P.; Engle, K. M. Catalytic Intermolecular Carboamination of Unactivated Alkenes via Directed Aminopalladation. J. Am. Chem. Soc. 2017, 139, 11261–11270.
- 41 Hu, Y.; Xie, Y. J.; Shen, Z. Q.; Huang, H. M. Palladium-Catalyzed Ring-Forming Aminoalkenylation of Alkenes with Aldehydes Initiated by Intramolecular Aminopalladation. Angew. Chem. Int. Ed. 2017, 56, 2473–2477.
- 42 Jin, Y.; Jing, Y.; Li, C.; Li, M.; Wu, W.; Ke, Z.; Jiang, H. Palladium-catalysed selective oxidative amination of olefins with Lewis basic amines. Nat. Chem. 2022, 14, 1118–1125.
- 43 Li, M.; Jin, Y.; Chen, Y.; Wu, W.; Jiang, H. Palladium-Catalyzed Oxidative Amination of Unactivated Olefins with Primary Aliphatic Amines. J. Am. Chem. Soc. 2023, 145, 9448–9453.
- 44 Åkermark, B.; Bäckvall, J. E.; Hegedus, L. S.; Zetterberg, K. Palladium-Promoted Addition of Amines to Isolated Double Bonds. J. Organometal. Chem. 1974, 72, 127–133.
- 45 Kohler, D. G.; Gockel, S. N.; Kennemur, J. L.; Waller, P. J.; Hull, K. L. Palladium-catalysed anti-Markovnikov selective oxidative amination. Nat. Chem. 2018, 10, 333–340.
- 46 Qi, X. T.; Kohler, D. G.; Hull, K. L.; Liu, P. Energy Decomposition Analyses Reveal the Origins of Catalyst and Nucleophile Effects on Regioselectivity in Nucleopalladation of Alkenes. J. Am. Chem. Soc. 2019, 141, 11892–11904.
- 47 Crawforth, C. M.; Burling, S.; Fairlamb, I. J. S.; Taylor, R. J. K.; Whitwood, A. C. Bromobis(triphenylphosphine)(N-succinimide)palladium(II) as a novel catalyst for Stille cross-coupling reactions. Chem. Commun. 2003, 2194–2195.
- 48 Crawforth, C. M.; Burling, S.; Fairlamb, I. J. S.; Kapdi, A. R.; Taylor, R. J. K.; Whitwood, A. C. Oxidative addition of N-halosuccinimides to palladium(0): the discovery of neutral palladium(II) imidate complexes, which enhance Stille coupling of allylic and benzylic halides. Tetrahedron 2005, 61, 9736–9751.
- 49 Haut, F.-L.; Habiger, C.; Wein, L. A.; Wurst, K.; Podewitz, M.; Magauer, T. Rapid Assembly of Tetrasubstituted Furans via Pummerer-Type Rearrangement. J. Am. Chem. Soc. 2021, 143, 1216–1223.
- 50 Yang, Z.-H.; Wang, Q.; Zhuo, S. P.; Xu, L.-P. Mechanistic Study on Palladium-Catalyzed Regioselective Oxidative Amination: Roles of Ammonium Salts. J. Org. Chem. 2020, 85, 6981–6991.
- 51 Whitfield, S. R.; Sanford, M. S. Reactivity of Pd(II) Complexes with Electrophilic Chlorinating Reagents: Isolation of Pd(IV) Products and Observation of C-Cl Bond-Forming Reductive Elimination. J. Am. Chem. Soc. 2007, 129, 15142–15143.
- 52 Powers, D. C.; Xiao, D. Y.; Geibel, M. A. L.; Ritter, T. On the Mechanism of Palladium-Catalyzed Aromatic C-H Oxidation. J. Am. Chem. Soc. 2010, 132, 14530–14536.
- 53 McCall, A. S.; Wang, H. W.; Desper, J. M.; Kraft, S. Bis-N-heterocyclic Carbene Palladium(IV) Tetrachloride Complexes: Synthesis, Reactivity, and Mechanisms of Direct Chlorinations and Oxidations of Organic Substrates. J. Am. Chem. Soc. 2011, 133, 1832–1848.
- 54CCDC 2247224 (4i), 1921764 (4x), 1921765 (6k) and 1921766 (6l) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 55 Song, L.; Luo, S. Z.; Cheng, J. P. Catalytic Intermolecular Haloamidation of Simple Alkenes with N-Halophthalimide as Both Nitrogen and Halogen Source. Org. Lett. 2021, 23, 5102–5106.




