Iron-Catalyzed Reductive C(aryl)—Si Cross-Coupling of Diaryl Ethers with Chlorosilanes
Corresponding Author
Pei Liu
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorBaowei Wu
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorCorresponding Author
Xuefeng Cong
Institute of Molecular Plus, Department of Chemistry and Haihe Laboratory of Sustainable Chemical Transformations, Tianjin University, 92 Weijin Road, Tianjin, 300072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jie Kong
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Pei Liu
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorBaowei Wu
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorCorresponding Author
Xuefeng Cong
Institute of Molecular Plus, Department of Chemistry and Haihe Laboratory of Sustainable Chemical Transformations, Tianjin University, 92 Weijin Road, Tianjin, 300072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jie Kong
School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi, 710072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
The reductive cross-coupling between C(aryl)—O and Si—Cl bonds is of much importance as a valuable strategy for the construction of C(aryl)—Si bonds but has remained a great challenge. Herein, we report a reductive cross-coupling of diaryl ethers and chlorosilanes via strong electrophilic C(aryl)—O and Si—Cl bonds cleavage by iron catalysis, which constitutes an efficient protocol for the synthesis of a range of functionalized arylsilanes. The combination of low cost FeCl2 as the precatalyst and iPrMgCl as the reductant shows high activity in the successive cleavage of unactivated C(aryl)—O bonds of diaryl ethers and strong electrophilic Si—Cl bonds of chlorosilanes, allowing their cross-coupling in a reductive fashion. The low-valent iron species generated in situ by reduction of FeCl2 with iPrMgCl was proposed, which prefers to initially cleavage the C(aryl)—O bond of diaryl ethers with the chelation help of an o-amide auxiliary.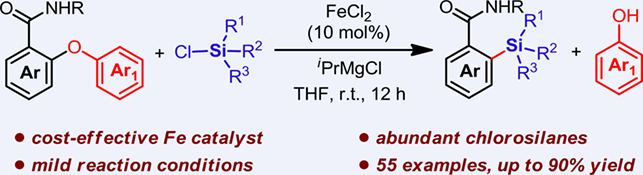
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300593-sup-0001-supinfo.pdfPDF document, 10.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
Yan, D.; Müller, T.; Bolte, M.; Auner, N. Origin of Photoluminescence in Organosilicon Compounds Containing Styrene Subunits. In Organosilicon Chemistry V: From Molecules to Materials, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003, pp. 139–144.
10.1002/9783527619924.ch21 Google Scholar
- 2 Franz, A. K.; Wilson, S. O. Organosilicon Molecules with Medicinal Applications. J. Med. Chem. 2013, 56, 388–405.
- 3 Ramesh, R.; Reddy, D. S. Quest for Novel Chemical Entities through Incorporation of Silicon in Drug Scaffolds. J. Med. Chem. 2018, 61, 3779–3798.
- 4
Hiyama, T.; Stang, P. J. Organosilicon Compounds in Cross-Coupling Reactions. In Metal-Catalyzed Cross-Coupling Reactions, John Wiley & Sons, New York, 1998, pp. 421–453.
10.1002/9783527612222.ch10 Google Scholar
- 5
Hiyama, T.; Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions, Wiley-VCH, 2019.
10.1002/9783527814787 Google Scholar
- 6 Keay, B. A. Product Subclass 33: Arylsilanes. In Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, Category 1, Organometallics, Vol. 4, Georg Thieme Verlag, Stuttgart, 2002, pp. 685–712.
- 7 Terao, J.; Kambe, N. Transition Metal Catalyzed Carbon-Silicon Bond Forming Reactions Using Chlorosilanes Promoted by Grignard Reagents. Chem. Rec. 2007, 7, 57–67.
- 8 Bähr, S.; Xue, W.; Oestreich, M. C(sp3)−Si Cross-Coupling. ACS Catal. 2019, 9, 16–24.
- 9 Korch, K. M.; Watson, D. A. Cross-Coupling of Heteroatomic Electrophiles. Chem. Rev. 2019, 119, 8192–8228.
- 10 Pang, X.; Shu, X.-Z. Nickel-Catalyzed Reductive Coupling of Chlorosilanes. Chem. Eur. J. 2023, 29, e202203362.
- 11 Murakami, K.; Hirano, K.; Yorimitsu, H.; Oshima, K. Silver-Catalyzed Transmetalation between Chlorosilanes and Aryl and Alkenyl Grignard Reagents for the Synthesis of Tetraorganosilanes. Angew. Chem. Int. Ed. 2008, 47, 5833–5835.
- 12 Vulovic, B.; Cinderella, A. P.; Watson, D. A. Palladium-Catalyzed Cross-Coupling of Monochlorosilanes and Grignard Reagents. ACS Catal. 2017, 7, 8113–8117.
- 13 Cinderella, A. P.; Vulovic, B.; Watson, D. A. Palladium-Catalyzed Cross-Coupling of Silyl Electrophiles with Alkylzinc Halides: A Silyl-Negishi Reaction. J. Am. Chem. Soc. 2017, 139, 7741–7744.
- 14 Kameo, H.; Yamamoto, H.; Ikeda, K.; Isasa, T.; Sakaki, S.; Matsuzaka, H.; García-Rodeja, Y.; Miqueu, K.; Bourissou, D. Fluorosilane Activation by Pd/Ni→Si−F→Lewis Acid Interaction: An Entry to Catalytic Sila-Negishi Coupling. J. Am. Chem. Soc. 2020, 142, 14039–14044.
- 15 Mcatee, J. R.; Martin, S. E. S.; Ahneman, D. T. A.; Johnson, K. A.; Watson, D. A. Preparation of Allyl and Vinyl Silanes via the Palladium-Catalyzed Silylation of Terminal Olefins: A Silyl-Heck Reaction. Angew. Chem. Int. Ed. 2012, 51, 3663–3667.
- 16 Mcatee, J. R.; Yap, G. P. A.; Watson, D. A. Rational Design of a Second Generation Catalyst for Preparation of Allylsilanes Using the Silyl-Heck reaction. J. Am. Chem. Soc. 2014, 136, 10166–10172.
- 17 Martin, S. E. S.; Watson, D. A. Preparation of Vinyl Silyl Ethers and Disiloxanes via the Silyl-Heck Reaction of Silyl Ditriflates. J. Am. Chem. Soc. 2013, 135, 13330–13333.
- 18 Matsumoto, K.; Huang, J.; Naganawa, Y.; Guo, H.; Beppu, T.; Sato, K.; Shimada, S.; Nakajima, Y. Direct Silyl-Heck Reaction of Chlorosilanes. Org. Lett. 2018, 20, 2481–2484.
- 19 Duan, J.; Wang, K.; Xu, G. L.; Kang, S.; Qi, L.; Liu, X. -Y.; Shu, X.-Z. Cross-Electrophile C(sp2)−Si Coupling of Vinyl Chlorosilanes. Angew. Chem. Int. Ed. 2020, 59, 23083–23088.
- 20 Zhao, Z.-Z.; Pang, X.; Wei, X.-X.; Liu, X.-Y., Shu, X.-Z. Nickel-Catalyzed Reductive C(sp2)−Si Coupling of Chlorohydrosilanes via Si−Cl Cleavage. Angew. Chem. Int. Ed. 2022, 61, e202200215.
- 21 Pan, Q.-Q.; Qi, L.; Pang, X.; Shu, X.-Z. Nickel-Catalyzed Cross- Electrophile 1,2-Silyl-Arylation of 1,3-Dienes with Chlorosilanes and Aryl Bromides. Angew. Chem. Int. Ed. 2023, 62, e202215703.
- 22 Zhang, L.; Oestreich, M. Nickel-Catalyzed, Reductive C(sp3)−Si Cross- Coupling of α-Cyano Alkyl Electrophiles and Chlorosilanes. Angew. Chem. Int. Ed. 2021, 60, 18587–18590.
- 23 Guan, W.; Lu, L.; Jiang, Q.; Gittens, A. F.; Wang, Y.; Novaes, L. F.; Klausen, R. S.; Lin, S. An Electrochemical Strategy to Synthesize Disilanes and Oligosilanes from Chlorosilanes. Angew. Chem. Int. Ed. 2023, 62, e202303592.
- 24 Duan, J.; Wang, Y.; Qi, L.; Guo, P.; Pang, X.; Shu, X.-Z. Nickel-Catalyzed Cross-Electrophile C(sp3)−Si Coupling of Unactivated Alkyl Bromides with Vinyl Chlorosilanes.Org. Lett. 2021, 23, 7855–7859.
- 25 Xing, M.; Cui, H.; Zhang, C. Nickel-Catalyzed Reductive Cross- Coupling of Alkyl Bromides and Chlorosilanes. Org. Lett. 2021, 23, 7645–7649.
- 26 Xue, W.; Shishido, R.; Oestreich, M. Bench-Stable Stock Solutions of Silicon Grignard Reagents: Application to Iron- and Cobalt-Catalyzed Radical C(sp3)−Si Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2018, 57, 12141–12145.
- 27 Mao, W.; Xue, W.; Irran, E.; Oestreich, M. Copper-Catalyzed Regio- and Enantioselective Addition of Silicon Grignard Reagents to Alkenes Activated by Azaaryl Groups. Angew. Chem. Int. Ed. 2019, 58, 10723–10726.
- 28 Zarate, C.; Nakajima, M.; Martin, R. A Mild and Ligand-Free Ni-Catalyzed Silylation via C−OMe Cleavage. J. Am. Chem. Soc. 2017, 139, 1191–1197.
- 29 Chu, C. K.; Liang, Y.; Fu, G. C. Silicon−Carbon Bond Formation via Nickel-Catalyzed Cross-Coupling of Silicon Nucleophiles with Unactivated Secondary and Tertiary Alkyl Electrophiles. J. Am. Chem. Soc. 2016, 138, 6404–6407.
- 30 Chen, S.; Guo, X.; Hou, H.; Geng, S.; Liu, Z.; He, Y.; Xue, X. -S.; Feng, Z. Thioethers as Dichotomous Electrophiles for Site-Selective Silylation via C−S Bond Cleavage. Angew. Chem. Int. Ed. 2023, 62, e202303470.
- 31 Cheng, C.; Hartwig, J. F. Catalytic Silylation of Unactivated C−H Bonds. Chem. Rev. 2015, 115, 8946–8975.
- 32 Shimada, M.; Yamanoi, Y.; Nishihara, H. Unusual Reactivity of Group 14 Hydrides Toward Organic Halides: Synthetic Studies and Application to Functional Materials. J. Synth. Org. Chem. Jpn. 2016, 74, 1098–1107.
- 33 Richter, S. C.; Oestreich, M. Emerging Strategies for C−H Silylation. Trends Chem. 2020, 2, 13–27.
- 34 Li, C.; Yang, S.; Zeng, X. Cross-Electrophile Silylation of Aryl Carboxylic Esters with Hydrochlorosilanes by SiH−Directed and Cr-Catalyzed Couplings. ACS Catal. 2023, 13, 12062–12073.
- 35 Tian, H.; Zhang, R.; Shi, L.; Zhao, C.; Wang, X. Divergent Synthesis of Organofluorinated Molecules from Titanium Mediated Deoxygenation of Free Alcohols. Chin. J. Chem. 2023, 41, 1783–1790.
- 36 Cornella, J.; Zarate, C.; Martin, R. Metal-catalyzed activation of ethers via C−O bond cleavage: a new strategy for molecular diversity. Chem. Soc. Rev. 2014, 43, 8081–8097.
- 37 Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C−O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726.
- 38 Pan, X.; Shu, X.-Z. Reductive Deoxygenative Functionalization of Alcohols by First-Row Transition Metal Catalysis. Chin. J. Chem. 2023, 41, 1637–1652.
- 39 Su, B.; Cao, Z.-C.; Shi, Z.-J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896.
- 40 Boit, T. B.; Bulger, A. S.; Dander, J. E.; Garg, N. K. Activation of C−O and C−N Bonds Using Non-Precious-Metal Catalysis. ACS Catal. 2020, 10, 12109–12126.
- 41 Zeng, H.; Qiu, Z.; Domínguez-Huerta, A.; Hearne, Z.; Chen, Z.; Li, C.-J. An Adventure in Sustainable Cross-Coupling of Phenols and Derivatives via Carbon−Oxygen Bond Cleavage. ACS Catal. 2017, 7, 510–519.
- 42 Bai, D.; Chen, J.; Zheng, B.; Li, X.; Chang J. Catalytic [3+3] Annulation of β-Ketoethers and Cyclopropenones via C(sp3)—O/C—C Bond Cleavage under Transition-Metal Free Conditions. Chin. J. Chem. 2021, 39, 2769–2773.
- 43 Cong, X.; Tang, H.; Zeng, X. Regio- and Chemoselective Kumada−Tamao−Corriu Reaction of Aryl Alkyl Ethers Catalyzed by Chromium under Mild Conditions. J. Am. Chem. Soc. 2015, 137, 14367–14372.
- 44 Liu, T.-X.; Yang, P.; Ma, N.; Li, X.; Wang, X.; Ma, J.; Zhang, G. Synthesis of [60]Fullerene-Fused Allylbenzofurans via Palladium-Catalyzed Migration Reaction. Chin. J. Chem. 2023, 41, 1733–1739.
- 45 Tobisu, M.; Takahira, T.; Morioka, T.; Chatani, N. Nickel-Catalyzed Alkylative Cross-Coupling of Anisoles with Grignard Reagents via C−O Bond Activation. J. Am. Chem. Soc. 2016, 138, 6711–6714.
- 46 Gong, L.; Li, C.; Yuan, F.; Liu, S.; Zeng, X. Chromium-Catalyzed Selective Borylation of Vinyl Triflates and Unactivated Aryl Carboxylic Esters with Pinacolborane. Org. Lett. 2022, 24, 3227–3231.
- 47 Tang, J.; Liu, L. L.; Yang, S.; Cong, X.; Luo, M.; Zeng, X. Chemoselective Cross-Coupling between Two Different and Unactivated C(aryl)−O Bonds Enabled by Chromium Catalysis. J. Am. Chem. Soc. 2020, 142, 7715–7720.
- 48 Xiong, Y.; Luo, M.; Zeng, X. Chromium-Catalyzed Three-Component Synthesis of Monofluoroalkenes from gem-Difluoroalkenes via C−O/C−H and C−F Bond Activation. Org. Lett. 2023, 25, 3120–3125.
- 49 Seki, R.; Hara, N.; Saito, T.; Nakao, Y. Selective C−O Bond Reduction and Borylation of Aryl Ethers Catalyzed by a Rhodium−Aluminum Heterobimetallic Complex. J. Am. Chem. Soc. 2021, 143, 6388–6394.
- 50 Li, C.; Ling, L.; Luo, Z.; Wang, S.; Zhang, X.; Zeng X. Deoxygenative Cross-Coupling of C(aryl)−O and C(amide)=O Electrophiles Enabled by Chromium Catalysis Using Bipyridine Ligand. ACS Catal. 2023, 13, 3120–3130.
- 51 Cong, X.; Zeng, X. Chromium-Catalyzed Cross-Coupling Reactions by Selective Activation of Chemically Inert Aromatic C−O, C−N, and C−H Bonds. Synlett 2021, 32, 1343–1353.
- 52 Zhang, J.; Zhang, Y.; Geng, S.; Chen, S.; Liu, Z.; Zeng, X.; He, Y.; Feng, Z. C−O Bond Silylation Catalyzed by Iron: A General Method for the Construction of Csp2−Si Bonds. Org. Lett. 2020, 22, 2669–2674.
- 53 Sergeev, A. G.; Hartwig, J. F. Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers. Science 2011, 332, 439–443.
- 54 Sergeev, A. G.; Webb, J. D.; Hartwig, J. F. A Heterogeneous Nickel Catalyst for the Hydrogenolysis of Aryl Ethers without Arene Hydrogenation. J. Am. Chem. Soc. 2012, 134, 20226–20229.
- 55 Ren, Y.; Yan, M.; Wang, J.; Zhang, Z. C.; Yao, K. Selective Reductive Cleavage of Inert Aryl C−O Bonds by an Iron Catalyst. Angew. Chem. Int. Ed. 2013, 52, 12674–12678.
- 56 Saper, N. I.; Hartwig, J. F. Mechanistic Investigations of the Hydrogenolysis of Diaryl Ethers Catalyzed by Nickel Complexes of N-Heterocyclic Carbene Ligands. J. Am. Chem. Soc. 2017, 139, 17667–17676.
- 57 Gao, F.; Webb, J. D.; Hartwig, J. F. Chemo- and Regioselective Hydrogenolysis of Diaryl Ether C−O Bonds by a Robust Heterogeneous Ni/C Catalyst: Applications to the Cleavage of Complex Lignin-Related Fragments. Angew. Chem. Int. Ed. 2016, 55, 1474–1478.
- 58 Sherry, B. D.; Fürstner, A. The Promise and Challenge of Iron- Catalyzed Cross Coupling. Acc. Chem. Res. 2008, 41, 1500–1511.
- 59 Bauer, I.; Knölker, H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387.
- 60 Cui, T.; Ye, C.-X.; Thelemann, J.; Jenisch, D.; Meggers, E. Enantioselective and Enantioconvergent Iron-Catalyzed C(sp3)−H Aminations to Chiral 2-Imidazolidinones. Chin. J. Chem. 2023, 41, 2065–2070.
- 61 Jing, K.; Chen, L.; Zhang, P.; Xu, S. Iridium-Catalyzed Site- and Enantioselective C(sp2)−H Borylation of Benzhydryl Ethers: Enantioselectivity Amplification by Kinetic Resolution Relay. Chin. J. Chem. 2023, 41, 2119–2124.
- 62 Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed C−H Bond Activation. Chem. Rev. 2017, 117, 9086–9139.
- 63 Wei, D.; Darcel, C. Iron Catalysis in Reduction and Hydrometalation Reactions. Chem. Rev. 2019, 119, 2550–2610.
- 64 Li, W.-D.; Chen, J.; Zhu, D.-Y.; Xia, J.-B. Fe-Catalyzed Pictet-Spengler- Type Cyclization via Selective Four-Electron Reductive Functionalization of CO2. Chin. J. Chem. 2020, 39, 614–620.
- 65 Rana, S.; Biswas, J. P.; Paul, S.; Paik, A.; Maiti, D. Organic synthesis with the most abundant transition metal−iron: from rust to multitasking catalysts. Chem. Soc. Rev. 2021, 50, 243–472.
- 66 Britton, L.; Docherty, H. J.; Nichol, S. G.; Dominey, P. A.; Thomas, P. S. Iron-Catalysed C(sp2)−H Borylation with Expanded Functional Group Tolerance. Chin. J. Chem. 2022, 40, 2875–2881.
- 67 Guo, J.; Cheng, Z.; Chen, J.; Chen, X.; Lu, Z. Iron- and Cobalt-Catalyzed Asymmetric Hydrofunctionalization of Alkenes and Alkynes. Acc. Chem. Res. 2021, 54, 2701–2716.
- 68 Nassar, Y.; Rodier, F.; Ferey, V.; Cossy, J. Cross-Coupling of Ketone Enolates with Grignard and Zinc Reagents with First-Row Transition Metal Catalysts. ACS Catal. 2021, 11, 5736–5761.
- 69 Xu, S.; Liu, G.; Huang, Z. Iron Catalyzed Isomerization of α-Alkyl Styrenes to Access Trisubstituted Alkenes. Chin. J. Chem. 2020, 39, 585–589.
- 70 Fürstner, A. Iron Catalyzed C−C-Bond Formation: From Canonical Cross Coupling to a Quest for New Reactivity. Bull. Chem. Soc. Jpn. 2021, 94, 666–677.
- 71 Li, B.-J.; Xu, L.; Wu, Z.-H.; Guan, B.-T.; Sun, C.-L., Wang, B.-Q.; Shi, Z.-J. Cross-Coupling of Alkenyl/Aryl Carboxylates with Grignard Reagent via Fe-Catalyzed C−O Bond Activation. J. Am. Chem. Soc. 2009, 131, 14656–14657.
- 72 Silberstein, A. L.; Ramgren, S. D.; Garg, N. K. Iron-Catalyzed Alkylations of Aryl Sulfamates and Carbamates. Org. Lett. 2012, 14, 3796–3799.
- 73 Agrawal, T.; Cook, S. P. Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards. Org. Lett. 2014, 16, 5080–5083.
- 74 Yu, D.-G.; Wang, X.; Zhu, R.-Y.; Luo, S.; Zhang, X.-B.; Wang, B.-Q.; Wang, L.; Shi, Z.-J. Direct Arylation/Alkylation/Magnesiation of Benzyl Alcohols in the Presence of Grignard Reagents via Ni-, Fe-, or Co-catalyzed sp3 C−O Bond Activation. J. Am. Chem. Soc. 2012, 134, 14638–14641.
- 75 Sun, C. L.; Fürstner, A. Formal Ring-Opening/Cross-Coupling Reactions of 2-Pyrones: Iron-Catalyzed Entry into Stereodefined Dienyl Carboxylates. Angew. Chem. Int. Ed. 2013, 52, 13071–13075.
- 76 Gärtner, D.; Stein, A. L.; Grupe, S.; Arp, J.; Wangelin, A. J. V. Iron-Catalyzed Cross-Coupling of Alkenyl Acetates. Angew. Chem. Int. Ed. 2015, 54, 10545–10549.
- 77 Iwasaki, T.; Akimoto, R.; Kuniyasu, H.; Kambe, N. Fe-Catalyzed Cross-Coupling Reaction of Vinylic Ethers with Aryl Grignard Reagents. Chem.-Asian J. 2016, 11, 2834–2837.
- 78 Rivera, A. C. P.; Still, R.; Frantz, D. E. Iron-Catalyzed Stereoselective Cross-Coupling Reactions of Stereodefined Enol Carbamates with Grignard Reagents. Angew. Chem. Int. Ed. 2016, 55, 6689–6693.
- 79
Chen, S.; Wang, Z.; Geng, S.; Zhu, H.; Liu, Z.; He, Y.; Peng, Q.; Feng, Z. Iron-Catalyzed Cross-Electrophile Coupling of Inert C−O Bonds with Alkyl Bromides. CCS Chem. 2022, 5, 1674–1685.
10.31635/ccschem.022.202202234 Google Scholar
- 80 Yoshikai, N.; Matsumoto, A.; Norinder, J.; Nakamura, E. Iron- Catalyzed Chemoselective ortho Arylation of Aryl Imines by Directed C−H Bond Activation. Angew. Chem. Int. Ed. 2009, 48, 2925–2928.
- 81 Ilies, L.; Konno, E.; Chen, Q.; Nakamura, E. Iron-Catalyzed ortho Monoarylation of Benzamide Derivatives. Asian J. Org. Chem. 2012, 1, 142–145.
- 82 Matsubara, T.; Asako, S.; Ilies, L.; Nakamura, E. Synthesis of Anthranilic Acid Derivatives through Iron-Catalyzed Ortho Amination of Aromatic Carboxamides with N-Chloroamines. J. Am. Chem. Soc. 2014, 136, 646–649.
- 83 Liu, P.; Hao, N.; Yang, D.; Wan, L.; Wang, T.; Zhang, T.; Zhou, R.; Cong, X.; Kong, J. Iron-catalyzed para-selective C−H silylation of benzamide derivatives with chlorosilanes. Org. Chem. Front. 2021, 8, 2863–2863.
- 84 Fürstner, A.; Leitner, A.; Méndez, M.; Krause, H. Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863.
- 85 Fürstner, A.; Martin, R.; Krause, H.; Seidel, G.; Goddard, R.; Lehmann, C. W. Preparation, Structure, and Reactivity of Nonstabilized Organoiron Compounds. Implications for Iron-Catalyzed Cross Coupling Reactions. J. Am. Chem. Soc. 2008, 130, 8773–8787.
- 86 Fürstner, A.; Leitner, A. Iron-Catalyzed Cross-Coupling Reactions of Alkyl-Grignard Reagents with Aryl Chlorides, Tosylates, and Triflates. Angew. Chem. Int. Ed. 2002, 41, 609–612.
- 87 Casitas, A.; Krause, H.; Goddard, R.; Fürstner, A. Elementary Steps of Iron Catalysis: Exploring the Links between Iron Alkyl and Iron Olefin Complexes for their Relevance in C−H Activation and C−C Bond Formation. Angew. Chem. Int. Ed. 2015, 54, 1521–1526.
- 88 Casitas, A.; Krause, H.; Lutz, S.; Fürstner, A. Ligand Exchange on and Allylic C−H Activation by Iron(0) Fragments: π-Complexes, Allyliron Species, and Metallacycles. Organometallics 2018, 37, 729–739.
- 89 Sun, Y.; Tang, H.; Chen, K.; Hu, L.; Yao, J.; Shaik, S.; Chen, H. Two-State Reactivity in Low-Valent Iron-Mediated C−H Activation and the Implications for Other First-Row Transition Metals. J. Am. Chem. Soc. 2016, 138, 3715–3730.
- 90 Xie, S.-J.; Cui, Y.-S.; Huang, Y.-F.; Liu, F.; Zhai, D.-D.; Shi Z.-J. Mechanistic Studies on Programmed C−O/C−H Activation with Valence-Adjusted Ti-Complexes. Chin. J. Chem. 2023, 41, 1015–1022.
- 91 Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed Directed C(sp2)−H and C(sp3)−H Functionalization with Trimethylaluminum. J. Am. Chem. Soc. 2015, 137, 7660–7663.
- 92 Beppu, T.; Sakamoto, K.; Nakajima, Y.; Matsumoto, K.; Sato, K. Shimada, S. Hydrosilane synthesis via catalytic hydrogenolysis of halosilanes using a metal-ligand bifunctional iridium catalyst. J. Organomet. Chem. 2018, 869, 75–80.
- 93 Shang, R.; Ilies, L.; Asako, S.; Nakamura, E. Iron-Catalyzed C(sp2)−H Bond Functionalization with Organoboron Compounds. J. Am. Chem. Soc. 2014, 136, 14349–14352.
- 94 Durin, G.; Berthet, J. C.; Nicolas, E.; Cantat, T. Unlocking the Catalytic Hydrogenolysis of Chlorosilanes into Hydrosilanes with Superbases. ACS Catal. 2021, 11, 10855–10861.




