Nickel-Catalyzed Regiodivergent Acylzincation of Styrenes with Organozincs and CO†
Chenglong Wang
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
‡ These authors contributed equally.
†Dedicated to the Special Issue of C1 Chemistry.
Search for more papers by this authorNing Liu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
‡ These authors contributed equally.
†Dedicated to the Special Issue of C1 Chemistry.
Search for more papers by this authorXianqing Wu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorJingping Qu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yifeng Chen
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorChenglong Wang
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
‡ These authors contributed equally.
†Dedicated to the Special Issue of C1 Chemistry.
Search for more papers by this authorNing Liu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
‡ These authors contributed equally.
†Dedicated to the Special Issue of C1 Chemistry.
Search for more papers by this authorXianqing Wu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorJingping Qu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yifeng Chen
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
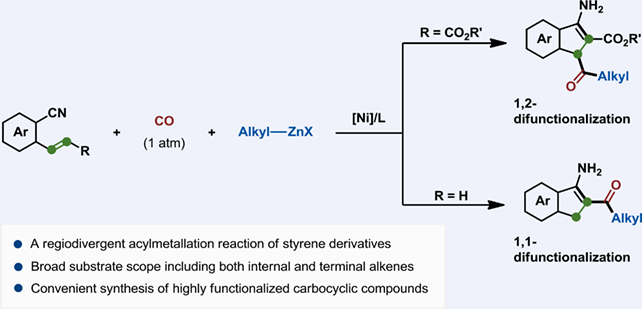
Transition metal-catalyzed carbometallation of unsaturated hydrocarbons constitutes one of the most efficient synthetic methodologies for the construction of C—C bond. Recently, the incorporation of organometallic reagent with the CO gas as a nucleophilic acyl synthon could enable the acylmetallation reaction, which greatly increases the horizon of carbometallation chemistry. Herein, we report a nickel-catalyzed regiodivergent acylzincation of o-cyano cinnamate ester and o-cyano styrene, in which the cyano moiety intramolecularly captures zinc intermediates to trigger the tandem cyclization process. This protocol features mild conditions, broad substrate scope and excellent functional group tolerance, thus affording a diverse array of highly functionalized carbocyclic compounds.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300590-sup-0001-Supinfo.pdfPDF document, 9.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340; (b) Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371–1394; (c) Derosa, J.; Apolinar, O.; Kang, T.; Tran, V. T.; Engle, K. M. Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkene. Chem. Sci. 2020, 11, 4287–4296; (d) Zhu, S.; Zhao, X.; Li, H.; Chu, L. Catalytic Three-Component Dicarbofuncationalization Reaction Involving Radical Capture by Nickel. Chem. Soc. Rev. 2021, 50, 10836–10856; (e) Gao, P.; Niu, Y.-J.; Yang, F.; Guo, L. N.; Duan, X.-H. Three-Component 1,2-Dicarbofunctionalization of Alkenes Involving Alkyl Radicals. Chem. Commun. 2022, 58, 730–746.
- 2(a) Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of Quaternary Stereocenters by Palladium Catalyzed Carbopalladation-Initiated Cascade Reactions. Angew. Chem. Int. Ed. 2019, 58, 1562–1573; (b) Ping, Y.; Song, H.; Kong, W. Recent Advances in Ni-Catalyzed Asymmetric Reductive Difunctionalization of Alkenes. Chin. J. Org. Chem. 2022, 42, 3302–3321.
- 3(a) Zhang, Z.-M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y.; Zhang, J. Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction. Angew. Chem. Int. Ed. 2019, 58, 14653–14659; (b) Wang, K.; Ding, Z.; Zhou, Z.; Kong, W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 12364–12368; (c) He, J.; Xue, Y.; Han, B.; Zhang, C.; Wang, Y.; Zhu, S. Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem. Int. Ed. 2020, 59, 2328–2332; (d) Li, Y.; Zhang, F.-P.; Wang, R.-H.; Qi, S.-L.; Luan, Y.-X.; Ye, M. Carbamoyl Fluoride-Enabled Enantioselective Ni-Catalyzed Carbocarbamoylation of Unactivated Alkenes. J. Am. Chem. Soc. 2020, 142, 19844–19849.
- 4(a) You, W.; Brown, M. K. Diarylation of Alkenes by a Cu-Catalyzed Migratory Insertion/Cross-Coupling Cascade. J. Am. Chem. Soc. 2014, 136, 14730–14733; (b) Cong, H.; Fu, G. C. Catalytic Enantioselective Cyclization/Cross-Coupling with Alkyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 3788–3791.
- 5(a) Brennfϋhrer, A.; Neumann, H.; Beller, M. Angew. Chem. Int. Ed. 2009, 48, 4114–4133; (b) Wu, X.-F.; Neumann, H.; Beller, M. Chem. Soc. Rev. 2011, 40, 4986–5009; (c) Peng, J.-B.; Wu, F.-P.; Wu, X.-F. Chem. Rev. 2019, 119, 2090–2127; (d) Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. Chem 2019, 5, 526–552.
- 6(a) Zhao, H.-Y.; Gao, X.; Zhang, S.; Zhang, X. Nickel-Catalyzed Carbonylation of Difluoroalkyl Bromides with Arylboronic acids. Org. Lett. 2019, 21, 1031–1036; (b) Cheng, R.; Zhao, H.-Y.; Zhang, S.; Zhang, X. Nickel-Catalyzed Carbonylation of Secondary Trifluoromethylated, Difluoromethylated, and Nonfluorinated Aliphatic Electrophiles with Arylboronic Acids under 1 atm of CO. ACS Catal. 2020, 10, 36–42; (c) Zhou, M.; Zhao, H.-Y.; Zhang, S.; Zhang, Y.; Zhang, X. Nickel-Catalyzed Four-Component Carbocarbonylation of Alkenes under 1 atm of CO. J. Am. Chem. Soc. 2020, 142, 18191–18199; (d) Cheng, R.; Sang, Y.; Gao, X.; Zhang, S.; Xue, X.-S.; Zhang, X. Highly ϒ-Selective Arylation and Carbonylative Arylation of 3-Bromo-3,3- Difluoropropene via Nickel Catalysis. Angew. Chem. Int. Ed. 2021, 60, 12386–12391; (e) Andersen, T. L.; Donslund, A. S.; Neumann, K. T.; Skrydstrup, T. Carbonylative Coupling of Alkyl Zinc Reagents with Benzyl Bromides Catalyzed by a Nickel/NN2 Pincer Ligand Complex. Angew. Chem. Int. Ed. 2018, 57, 800–804; (f) Donslund, A. S.; Neumann, K. T.; Corneliussen, N. P.; Grove, E. K.; Herbstritt, D.; Daasbjerg, K.; Skrydstrup, T. Access to β-Ketonitriles through Nickel-Catalyzed Carbonylative Coupling of α-Bromonitriles with Alkylzinc Reagents. Chem. Eur. J. 2019, 25, 9856–9860; (g) Ravn, A. K.; Vilstrup, M. B. T.; Noerby, P.; Nielsen, D. U.; Daasbjerg, K.; Skrydstrup, T. Carbon Isotope Labeling Strategy for β-Amino Acid Derivatives via Carbonylation of Azanickellacycle. J. Am. Chem. Soc. 2019, 141, 11821–11826; (h) Zhao, X.; Feng, X.; Chen, F.; Zhu, S.; Qing, F.-L.; Chu, L. Divergent Aminocarbonylations of Alkynes Enabled by Photoredox/Nickel Dual Catalysis. Angew. Chem. Int. Ed. 2021, 60, 26511–26517; (i) Yu, R.; Cai, S.-Z.; Li, C.; Fang, X. Nickel-Catalyzed Asymmetric Hydroaryloxy- and Hydroalkoxycarbonylation of Cyclopropenes. Angew. Chem. Int. Ed. 2022, 61, e202200733; (j) Zhang, Y.; Zhang, Z.; Zhu, S.; Chu, L. Chin. J. Org. Chem. 2023, 43, 1023–1035; (k) Chen, X.; Chen, G.; Lian, Z. Recent Advances in Nickel Catalyzed Carbonylative Reactions via the insertion of Carbon Monoxide. Chin. J. Chem. 2024, 42, 177–189.
- 7(a) Peng, J.-B.; Wu, F.-P.; Xu, C.; Qi, X.; Ying, J.; Wu, X.-F. Nickel- Catalyzed Carbonylative Synthesis of Functionalized Alkyl Iodides. iScience 2018, 8, 175–182; (b) Cheung, C. W.; Ploeger, M. L.; Hu, X. Amide Synthesis via Nickel-Catalysed Reductive Aminocarbonylation of Aryl Halides with Nitroarenes. Chem. Sci. 2018, 9, 655–659; (c) Huo, Y.-W.; Yao, L.; Qi, X.; Wu, X.-F. Nickel-Catalyzed Reductive Aminocarbonylation of Vinyl Triflates with Nitro Compounds for the Synthesis of α,β-Unsaturated Amides. Org. Chem. Front. 2021, 8, 6974–6978; (d) Wang, L.-C.; Chen, B.; Zhang, Y.; Wu, X.-F. Nickel- Catalyzed Four-Component Carbonylation of Ethers and Olefins: Direct Access to γ-Oxy Esters and Amides. Angew. Chem. Int. Ed. 2022, 61, e202207970.
- 8(a) Shi, R.; Hu, X. From Alkyl Halides to Ketones: Nickel-Catalyzed Reductive Carbonylation Utilizing Ethyl Chloroformate as the Carbonyl Source. Angew. Chem. Int. Ed. 2019, 58, 7454–7458; (b) Chen, J.; Zhu, S. Nickel-Catalyzed Multicomponent Coupling: Synthesis of α-Chiral Ketones by Reductive Hydrocarbonylation of Alkenes. J. Am. Chem. Soc. 2021, 143, 14089–14096; (c) Jiang, Y.; Yang, K.; Wei, Y.; Wang, Q.; Li, S. J.; Lan, Y.; Koh, M. J. Catalytic Multicomponent Synthesis of C-Acyl Glycosides by Consecutive Cross-Electrophile Couplings. Angew. Chem. Int. Ed. 2022, 61, e202211043; (d) Chen, H.; Yue, H.; Zhu, C.; Rueping, M. Reactivity in Nickel-Catalyzed Multi- component Sequential Reductive Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2022, 61, e202204144; (e) Chen, J.; Deng, G.; Wang, Y.; Zhu, S. Facile Synthesis of Chiral α-Hydroxy Ketones by a Ni-Catalyzed Multicomponent Hydrometallation–CO Insertion–Enantioconvergent Alkylation Cascade. Chin. J. Chem. 2023, 41, 294–300.
- 9(a) Xie, P.; Xie, Y.; Qian, B.; Zhou, H.; Xia, C.; Huang, H. Palladium- Catalyzed Oxidative of Benzylic C-H Bonds via Nondirected C(sp3)–H Activation. J. Am. Chem. Soc. 2012, 134, 9902–9905; (b) Ding, Y.; Huang, H. Palladium-Catalyzed [4+1+1] Cycloaddition for the Direct Synthesis of N-Substituted Quinazoline-2,4(1H,3H)-diones. Chin. J. Org. Chem. 2021, 41, 1757–1758; (c) Ding, Y.; Huang, R.; Zhang, W.; Huang, H. Nickel-Catalyzed Oxidative Carbonylation of Alkylarenes to Arylacetic Acids. Org. Lett. 2022, 24, 7972–7977; (d) Ding, Y.; Wu, J.; Huang, H. Carbonylative Formal Cycloaddition between Alkylarenes and Aldimines Enabled by Palladium-Catalyzed Double C–H Bond Activation. J. Am. Chem. Soc. 2023, 145, 4982–4988;
- 10(a) Chen, M.; Wang, X.; Yang, P.; Kou, X.; Ren, Z.-H.; Guan, Z.-H. Palladium-Catalyzed Enantioselective Heck Carbonylation with a Monodentate Phosphoramidite Ligand: Asymmetric Synthesis of (+)-Physostigmine, (+)-Physovenine, and (+)-Folicanthine. Angew. Chem. Int. Ed. 2020, 59, 12199–12205; (b) Yao, Y.-H.; Zou, X.-J.; Wang, Y.; Yang, H.-Y.; Ren, Z.-H.; Guan, Z.-H. Palladium-Catalyzed Asymmetric Markovnikov Hydroxycarbonylation and Hydroalkoxycarbonylation of Vinyl Arenes: Synthesis of 2-Arylpropanoic Acids. Angew. Chem. Int. Ed. 2021, 60, 23117–23122; (c) Yao, Y.-H.; Yang, H.-Y.; Chen, M.; Wu, F.; Xu, X.-X.; Guan, Z.-H. Asymmetric Markovnikov Hydroaminocarbonylation of Alkenes Enabled by Palladium-Monodentate Phosphoramidite Catalysis. J. Am. Chem. Soc. 2021, 143, 85–91; (d) Yang, H.-Y.; Yao, Y.-H.; Chen, M.; Ren, Z.-H.; Guan, Z.-H. Palladium-Catalyzed Markovnikov Hydroaminocarbonylation of 1,1-Disubstituted and 1,1,2-Trisubstituted Alkenes for Formation of Amides with Quaternary Carbon. J. Am. Chem. Soc. 2021, 143, 7298–7305; (e) Chen, G.; Zhou, R.; Zhang, X.; Xiao, X.; Kramer, S.; Cheng, G.-J.; Lian, Z. Carbonylative Cross-Electrophile Coupling between Aryl Bromides and Aryl Triflates Enabled by Palladium and Rhodium Cooperative Catalysis and CO as Reductant. ACS Catal. 2022, 12, 14582–14591.
- 11(a) Weng, Y.; Zhang, Y.; Turlik, A.; Wu, X.; Li, H.; Fei, F.; Yao, Y.; Wang, C.; Guo, Z.; Qu, J.; Houk, K. N.; Chen, Y. Nickel-Catalysed Regio- and Stereoselective Acylzincation of Unsaturated Hydrocarbons with Organozincs and CO. Nat. Synth. 2023, 2, 261–274; (b) Wu, X.; Wang, C.; Liu, N.; Qu, J.; Chen, Y. Nickel-Catalyzed Acylzincation of Allenes with Organozincs and CO. Nat. Commun. 2023, 14, 6960.
- 12
Tamaru, Y. Modern Organonickel Chemistry, Wiley-VCH, Weinheim, Germany, 2005.
10.1002/3527604847 Google Scholar
- 13(a) Larock, R. C. In Comprehensive Organic Transformations: A Guide to Functional Group Preparations, Wiley-VCH, Weinheim, 1989, pp. 819–995; (b) Hsieh, J.-C. Transition-metal-catalyzed addition/cyclization reactions of the C-N multiple bonds containing species. Chem. Rec. 2021, 21, 3370–3381.
- 14(a) Chun, Y. S.; Xuan, Z.; Kim, J. H.; Lee, S.-g. An Expedient and Divergent Tandem One-Pot Synthesis of Pyrimidin-2,4-diones Using the Blaise Reaction Intermediate. Org. Lett. 2013, 15, 3162–3165; (b) Shin, U. S.; Joo, S.-R.; Kim, S.-H. Coupling Reactions of Zinc Amide Enolates with Nitriles: Preparation of β-Keto and β-Amino Amides. Bull. Korean Chem. Soc. 2015, 36, 2565–2568.
- 15(a) Weng, Y.; Zhang, C.; Tang, Z.; Shrestha, M.; Huang, W.; Qu, J.; Chen, Y. Nickel-Catalyzed Allylic Carbonylative Coupling of Alkyl Zinc Reagents with tert-butyl Isocyanide. Nat. Commun. 2020, 11, 392; (b) Huang, W.; Wang, Y.; Weng, Y.; Qu, J.; Chen, Y. Nickel-Catalyzed Formal Aminocarbonylation of Unactivated Alkyl Iodides with Isocyanides. Org. Lett. 2020, 22, 3245–3250; (c) Wang, Y.; Huang, W.; Wang, C.; Qu, J.; Chen, Y. Nickel-Catalyzed Formal Aminocarbonylation of Secondary Benzyl Chlorides with Isocyanides. Org. Lett. 2020, 22, 4245–4249; (d) Wang, C.; Wu, L.; Xu, W.; He, F.; Qu, J.; Chen, Y. Palladium-Catalyzed Secondary Benzylic Imidoylative Reactions. Org. Lett. 2020, 22, 6954–6959; (e) Liu, N.; Wu, X.; Wang, C.; Qu, J.; Chen, Y. Nickel-Catalyzed Alkoxycarbonylation of Aryl Iodides with 1 atm CO. Chem. Commun. 2022, 58, 4643–4646; (f) Hou, L.; Huang, W.; Wu, X.; Qu, J.; Chen, Y. Nickel-Catalyzed Carbonylation of Cyclopropanol with Benzyl Bromide for Multisubstituted Cyclopentenone Synthesis. Org. Lett. 2022, 24, 2699–2704; (g) Wang, C.; Wu, X.; Li, H.; Qu, J.; Chen, Y. Carbonylative Cross-Coupling Reaction of Allylic Alcohols and Organoalanes with 1 atm CO Enabled by Nickel Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202210484; (h) Liu, N.; Wu, X.; Wang, C.; Qu, J.; Chen, Y. Nickel-Catalyzed Aminocarbonylation of Aryl Iodides with 1 atm CO. Chem. Asian J. 2023, 18, e202201061.
- 16(a) Wu, X.; Qu, J.; Chen, Y. Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam. J. Am. Chem. Soc. 2020, 142, 15654–15660;
(b) Wu, X.; Luan, B.; Zhao, W.; He, F.; Wu, X.-Y.; Qu, J.; Chen, Y. Catalytic Desymmetric Dicarbofunctionalization of Unactivated Alkenes. Angew. Chem. Int. Ed. 2022, 61, e2021115983;
(c) Wu, X.; Turlik, A.; Luan, B.; He, F.; Qu, J.; Houk, K. N.; Chen, Y. Nickel-Catalyzed Enantioselective Reductive Alkyl-Carbamoylation of Internal Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202207536;
(d) He, F.; Hou, L.; Wu, X.; Ding, H.; Qu, J.; Chen, Y. Enantioselective Synthesis of α-Alkenylated γ-Lactam Enabled by Ni-Catalyzed 1,4-Arycarbamoylation of 1,3-Dienes. CCS Chem. 2023, 5, 341–349;
(e) Zhang, C.; Wu, X.; Xia, T.; Qu, J.; Chen, Y. Ni-catalyzed Carbamoylation of Unactivated Alkenes for Stereoselective Construction of Six-Membered Lactams. Nat. Commun. 2022, 13, 5964;
(f) Wu, X.; Li, H.; He, F.; Qu, J.; Chen, Y. Nickel/Quinim Enabled Asymmetric Carbamoyl-Acylation of Unactivated Alkenes. Chin. J. Chem. 2023, 41, 1673–1678;
(g) Wu, L.; Wu, X.; Qu, J.; Chen, Y. Exploration of Quinim Ligand in Ni-catalyzed Enantioselective Reductive Carbamoyl-Alkylation of Alkene. Chin. J. Org. Chem. 2023, DOI: https://doi.org/10.6023/cjoc202306006.
10.6023/cjoc202306006 Google Scholar




