Recent Advances in Deuteration Reactions†
Hao Li
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorMuhammad Shabbir
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wu Li
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Aiwen Lei
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorHao Li
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorMuhammad Shabbir
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wu Li
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Aiwen Lei
Institute for Advanced Studies (IAS), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to Prof. Xiyan Lu for his outstanding contributions to organometallic chemistry and catalysis.
Comprehensive Summary
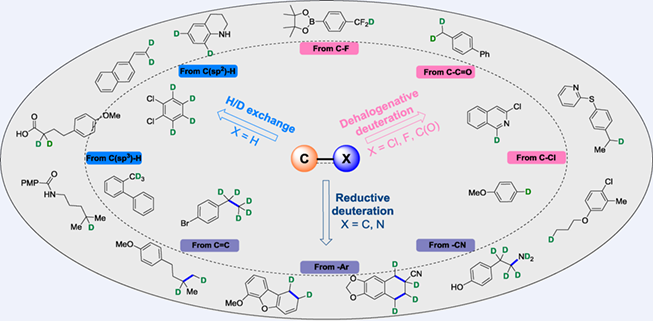
The deuteration of organic compounds has attracted more attentions in recent years for the potential applications in new drug discovery and synthetic chemistry. For this purpose, many efficient deuterium labeling methodologies have been developed, including hydrogen isotope exchange (HIE), reductive deuteration, and dehalogenative deuteration that allow for the synthesis of selectively deuterated compounds. In the last few years, great breakthroughs in selective isotope labeling have been achieved and the interest in new methodologies for the deuteration of organic molecules is rising. In this review, we summarized the recent developments in the selective deuteration of organic molecules since 2021. Several types of key processes in deuterium incorporation reactions, including H/D exchange, reductive deuteration and dehalogenative deuteration, are introduced and discussed.
Key Scientists
In the 2000s, Derdau and Atzrodt's group have made great contributions to the directing group assisted noble-metal catalyzed hydrogen isotope exchange of arenes and applications of labeled compounds. During the same period, Sajiki and co-workers completed a series of deuteration reactions by heterogeneous platinum-group metal catalysts. Since 2015, Gregory Pieters and co-workers have developed ruthenium catalysts for selective hydrogen isotope exchange. In 2016, Chirik's group achieved hydrogen isotope exchange in the presence of a homogeneous iron complex. David MacMillan and coworkers have made breakthroughs in photocatalyzed HIE reactions for α-amino C(sp3)–H bonds. From 2020, a series of nanoelectrodes were designed for selective dehalogenative deuterations and reductive deuteration of unactivated unsaturated bonds by Zhang's group. Recently, Beller's group developed several efficient strategies for isotopic labeling using heterogeneous earth-abundant catalysts. Our review summarized the latest and important developments since 2021.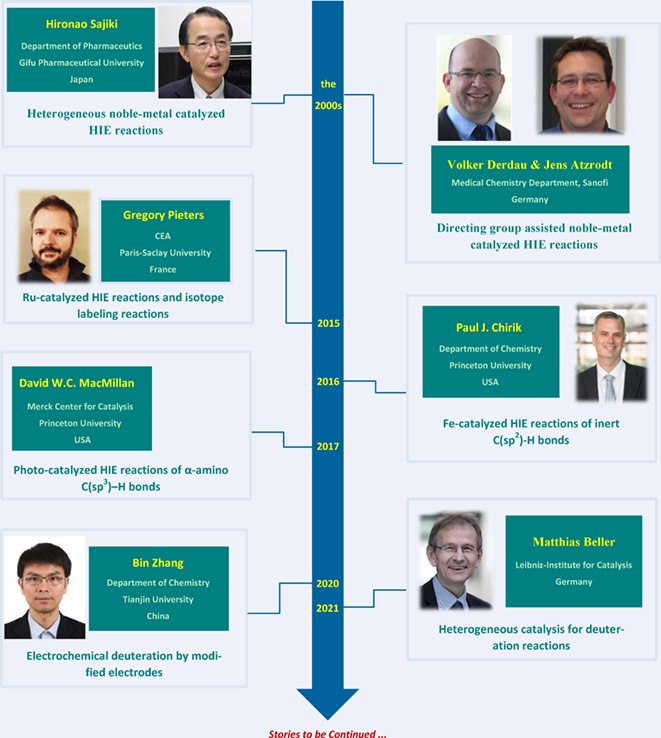
References
- 1 Urey, H. C.; Brickwedde, F. G.; Murphy, G. M. A Hydrogen Isotope of Mass 2 and its Concentration. Phys. Rev. 1932, 40, 1–15.
- 2 Smith, J. A.; Wilson, K. B.; Sonstrom, R. E.; Kelleher, P. J.; Welch, K. D.; Pert, E. K.; Westendorff, K. S.; Dickie, D. A.; Wang, X.; Pate, B. H.; Harman, W. D. Preparation of Cyclohexene Isotopologues and Stereoisotopomers from Benzene. Nature 2020, 581, 288–293.
- 3
Wiberg, K. B. The Deuterium Isotope Effect. Chem. Rev. 2002, 55, 713–743.
10.1021/cr50004a004 Google Scholar
- 4 Chandra Mouli, H. M.; Vinod, A.; Kumari, S.; Tiwari, A. K.; Kathiravan, M. K.; Ravichandiran, V.; Peraman, R. Deuterated Driven New Chemical Entities: An Optimistic Way to Improve Therapeutic Efficacy. Bioorg. Chem. 2023, 135. DOI: https://doi.org/10.1016/j.bioorg.2023.106490.
- 5 Di Martino, R. M. C.; Maxwell, B. D.; Pirali, T. Deuterium in Drug Discovery: Progress, Opportunities and Challenges. Nat. Rev. Drug Discov. 2023, 22, 562–584.
- 6 Schmidt, C. First Deuterated Drug Approved. Nat. Biotechnol. 2017, 35, 493–494.
- 7 Keam, S. J.; Duggan, S. Donafenib: First Approval. Drugs 2021, 81, 1915–1920.
- 8 Liu, C.; Zi, X.; Li, H. M.; Gao, K. G.; Hong, J.; Tao, J.; Yao, Z. S. Anisotropic Deuteration Effects on a Molecular Ferroelectric. Matter 2023, 6, 1639–1653.
- 9 Haidar, Y.; Konermann, L. Effects of Hydrogen/Deuterium Exchange on Protein Stability in Solution and in the Gas Phase. J. Am. Soc. Mass Spectrom. 2023, 34, 1447–1458.
- 10 Atzrodt, J.; Derdau, V.; Kerr, W. J.; Reid, M. C-H Functionalisation for Hydrogen Isotope Exchange. Angew. Chem. Int. Ed. 2018, 57, 3022–3047.
- 11 Atzrodt, J.; Derdau, V.; Fey, T.; Zimmermann, J. The Renaissance of H/D Exchange. Angew. Chem. Int. Ed. 2007, 46, 7744–7765.
- 12 Kopf, S.; Bourriquen, F.; Li, W.; Neumann, H.; Junge, K.; Beller, M. Recent Developments for the Deuterium and Tritium Labeling of Organic Molecules. Chem. Rev. 2022, 122, 6634–6718.
- 13 Prakash, G.; Paul, N.; Oliver, G. A.; Werz, D. B.; Maiti, D. C–H Deuteration of Organic Compounds and Potential Drug Candidates. Chem. Soc. Rev. 2022, 51, 3123–3163.
- 14 Li, N.; Li, Y. T.; Wu, X. P.; Zhu, C. J.; Xie, J. Radical Deuteration. Chem. Soc. Rev. 2022, 51 , 6291–6306.
- 15 Zhou, R.; Ma, L.; Yang, X.; Cao, J. Recent Advances in Visible-Light Photocatalytic Deuteration Reactions. Org. Chem. Front. 2021, 8, 426–444.
- 16 Norcott, P. L. Current Electrochemical Approaches to Selective Deuteration. Chem. Commun. 2022, 58, 2944–2953.
- 17 Beller, M.; Junge, K.; Bourriquen, F. Homogenous Iron-Catalysed Deuteration of Electron-Rich Arenes and Heteroarenes. Synlett 2022, 34, 332–336.
- 18 Yamada, T.; Arai, K.; Kikuchi, R.; Okamoto, S. Deuteration of Indole Compounds: Synthesis of Deuterated Auxins, Indole-3-acetic Acid-d5 and Indole-3-butyric Acid-d5. ACS Omega 2021, 6, 19956–19963.
- 19 Li, H.; Lai, Z.; Peng, M.; Ning, L.; Dong, Q.; Hou, Y.; An, J. One-Pot Sequential Hydrogen Isotope Exchange/Reductive Deuteration for the Preparation of α, β-Deuterated Alcohols Using Deuterium Oxide. Org. Lett. 2022, 24, 5319–5323.
- 20 Kopf, S.; Neumann, H.; Beller, M. Manganese-Catalyzed Selective C–H Activation and Deuteration by Means of a Catalytic Transient Directing Group Strategy. Chem. Commun. 2021, 57, 1137–1140.
- 21 Kramp, H.; Weck, R.; Sandvoss, M.; Sib, A.; Mencia, G.; Fazzini, P. F.; Chaudret, B.; Derdau, V. In Situ Generated Iridium Nanoparticles as Hydride Donors in Photoredox-Catalyzed Hydrogen Isotope Exchange Reactions with Deuterium and Tritium Gas. Angew. Chem. Int. Ed. 2023, 62, e2023089.
- 22 He, T.; Klare, H. F. T.; Oestreich, M. Perdeuteration of Deactivated Aryl Halides by H/D Exchange under Superelectrophile Catalysis. J. Am. Chem. Soc. 2022, 144, 4734–4738.
- 23 Tortajada, A.; Hevia, E. Perdeuteration of Arenes via Hydrogen Isotope Exchange Catalyzed by the Superbasic Sodium Amide Donor Species NaTMP·PMDETA. J. Am. Chem. Soc. 2022, 144, 20237–20242.
- 24 Li, W.; Rabeah, J.; Bourriquen, F.; Yang, D.; Kreyenschulte, C.; Rockstroh, N.; Lund, H.; Bartling, S.; Surkus, A.-E.; Junge, K.; Brückner, A.; Lei, A.; Beller, M. Scalable and Selective Deuteration of (Hetero)Arenes. Nat. Chem. 2022, 14, 334–341.
- 25 Jia, Z.; Luo, S. Visible Light Promoted Direct Deuteration of Alkenes via Co(III)–H Mediated H/D Exchange. CCS Chem. 2023, 5, 1069–1076.
- 26 Qian, P.; Zhang, S.; Luo, F.; Wang, J.; Zhang, X.; Liu, X.; Chen, X.; Wang, W.; Chen, X. Site-Selective Deuteration at the α-Position of Enals by an Amine and Bis(Phenylsulfonyl)Methane co-Catalyzed H/D Exchange Reaction. Chem. Commun. 2022, 58, 11458–11461.
- 27 Du, H. Z.; Fan, J. Z.; Wang, Z. Z.; Strotman, N. A.; Yang, H.; Guan, B. T. Cesium Amide-Catalyzed Selective Deuteration of Benzylic C-H Bonds with D2 and Application for Tritiation of Pharmaceuticals. Angew. Chem. Int. Ed. 2022, 62, e202214461.
- 28 Yang, H.; Huang, Z.; Lehnherr, D.; Lam, Y. H.; Ren, S.; Strotman, N. A. Efficient Aliphatic Hydrogen-Isotope Exchange with Tritium Gas Through the Merger of Photoredox and Hydrogenation Catalysts. J. Am. Chem. Soc. 2022, 144, 5010–5022.
- 29 Kang, Q. K.; Li, Y.; Chen, K.; Zhu, H.; Wu, W. Q.; Lin, Y.; Shi, H. Rhodium-Catalyzed Stereoselective Deuteration of Benzylic C-H Bonds via Reversible η6-coordination. Angew. Chem. Int. Ed. 2022, 61, e202117381.
- 30 Doyon, T. J.; Buller, A. R. Site-Selective Deuteration of Amino Acids Through Dual-Protein Catalysis. J. Am. Chem. Soc. 2022, 144, 7327–7336.
- 31
Tanaka, T.; Koga, Y.; Honda, Y.; Tsuruta, A.; Matsunaga, N.; Koyanagi, S.; Ohdo, S.; Yazaki, R.; Ohshima, T. Ternary Catalytic α-Deuteration of Carboxylic Acids. Nat. Synth. 2022, 1, 824–830.
10.1038/s44160-022-00139-9 Google Scholar
- 32 Chen, Q.; Liu, Q.; Xiao, J.; Leng, X. B.; Deng, L. Catalytic Method for the Synthesis of Deuterium-Labeled N-Heterocyclic Carbenes Enabled by a Coordinatively Unsaturated Ruthenium N-Heterocyclic Carbene Catalyst. J. Am. Chem. Soc. 2021, 143, 19956–19965.
- 33 Li, N.; Li, J.; Qin, M.; Li, J.; Han, J.; Zhu, C.; Li, W.; Xie, J. Highly Selective Single and Multiple Deuteration of Unactivated C(sp3)-H Bonds. Nat. Commun. 2022, 13, 4224.
- 34 Vang, Z. P.; Hintzsche, S. J.; Clark, J. R. Catalytic Transfer Deuteration and Hydrodeuteration: Emerging Techniques to Selectively Transform Alkenes and Alkynes to Deuterated Alkanes. Chem. Eur. J. 2021, 27, 9988–10000.
- 35 Suzuki, A.; Kamei, Y.; Yamashita, M.; Seino, Y.; Yamaguchi, Y.; Yoshino, T.; Kojima, M.; Matsunaga, S. Photocatalytic Deuterium Atom Transfer Deuteration of Electron-Deficient Alkenes with High Functional Group Tolerance. Angew. Chem. Int. Ed. 2022, 62, e202214433.
- 36 Kang, W. J.; Li, B.; Duan, M.; Pan, G.; Sun, W.; Ding, A.; Zhang, Y.; Houk, K. N.; Guo, H. Discovery of a Thioxanthone–TfOH Complex as a Photoredox Catalyst for Hydrogenation of Alkenes Using p-Xylene as Both Electron and Hydrogen Sources. Angew. Chem. Int. Ed. 2022, 61, e202211562.
- 37 Kurimoto, A.; Sherbo, R. S.; Cao, Y.; Loo, N. W. X.; Berlinguette, C. P. Electrolytic Deuteration of Unsaturated Bonds without Using D2. Nat. Catal. 2020, 3, 719–726.
- 38 Gao, J.; Ma, R.; Feng, L.; Liu, Y.; Jackstell, R.; Jagadeesh, R. V.; Beller, M. Ambient Hydrogenation and Deuteration of Alkenes Using a Nanostructured Ni-Core–Shell Catalyst. Angew. Chem. Int. Ed. 2021, 60, 18591–18598.
- 39 Wu, X. Y.; Gannett, C. N.; Liu, J. J.; Zeng, R.; Novaes, L. F. T.; Wang, H. S.; Abruna, H. D.; Lin, S. Intercepting Hydrogen Evolution with Hydrogen-Atom Transfer: Electron-Initiated Hydrofunctionalization of Alkenes. J. Am. Chem. Soc. 2022, 144, 17783–17791.
- 40 Li, A.; Song, X.; Ren, Q.; Bao, P.; Long, X.; Huang, F.; Yuan, L.; Zhou, J. S.; Qin, X. Cobalt-Catalyzed Asymmetric Deuteration of α-Amidoacrylates for Stereoselective Synthesis of α,β-Dideuterated α-Amino Acids. Angew. Chem. Int. Ed. 2023, 62, e202301091.
- 41 Luo, J.; Lu, L.; Montag, M.; Liang, Y.; Milstein, D. Hydrogenative Alkene Perdeuteration Aided by a Transient Cooperative Ligand. Nat. Chem. 2023. DOI: 10.1038/s41557–023–01313-y.
- 42 Zhang, J. J.; Muck-Lichtenfeld, C.; Studer, A. Photocatalytic Phosphine-Mediated Water Activation for Radical Hydrogenation. Nature 2023, 619, 506–513.
- 43 Yang, K.; Feng, T.; Qiu, Y. Organo-Mediator Enabled Electrochemical Deuteration of Styrenes. Angew. Chem. Int. Ed. 2023, 62, e20231280.
- 44 Shi, Q.; Xu, M.; Chang, R.; Ramanathan, D.; Penin, B.; Funes-Ardoiz, I.; Ye, J. Visible-Light Mediated Catalytic Asymmetric Radical Deuteration at Non-Benzylic Positions. Nat. Commun. 2022, 13, 4453.
- 45 Qiu, J. Y.; Zeng, W. L.; Xie, H.; Wang, M. Y.; Li, W. Chemoselective 1,2-Reduction and Regiodivergent Deuteration of Chromium-Bound Arenes. Angew. Chem. Int. Ed. 2023, 62, e202218961.
- 46 Li, R.; Wu, Y.; Wang, C.; He, M.; Liu, C.; Zhang, B. One-pot H/D Exchange and Low-Coordinated Iron Electrocatalyzed Deuteration of Nitriles in D2O to α,β-Deuterio Aryl Ethylamines. Nat. Commun. 2022, 13, 5951.
- 47 Mazzucato, M.; Isse, A. A.; Durante, C., Dissociative Electron Transfer Mechanism and Application in the Electrocatalytic Activation of Organic Halides. Curr. Opin. Electrochem. 2023, 39, 101254.
- 48 Lu, L. J.; Li, H.; Zheng, Y. F.; Bu, F. X.; Lei, A. W. Facile and Economical Electrochemical Dehalogenative Deuteration of (Hetero)Aryl Halides. CCS Chem. 2021, 3 , 2669–2675.
- 49 Li, Y.; Ye, Z.; Lin, Y. M.; Liu, Y.; Zhang, Y.; Gong, L. Organophotocatalytic Selective Deuterodehalogenation of Aryl or Alkyl Chlorides. Nat. Commun. 2021, 12, 2894.
- 50 Wood, D.; Lin, S. Deuterodehalogenation Under Net Reductive or Redox-Neutral Conditions Enabled by Paired Electrolysis. Angew. Chem. Int. Ed. 2023, 62, e202218858.
- 51 Box, J. R.; Avanthay, M. E.; Poole, D. L.; Lennox, A. J. J. Electronically Ambivalent Hydrodefluorination of Aryl-CF3 Groups Enabled by Electrochemical Deep-Reduction on a Ni Cathode. Angew. Chem. Int. Ed. 2023, 62, e202218195.
- 52 Sheng, J.; Cheng, X. Electrochemical Mono-Deuterodefluorination of Trifluoromethyl Aromatic Compounds with Deuterium Oxide. CCS Chem. 2023. DOI: https://doi.org/10.31635/ccschem.023.202302835.
- 53 Mészáros, R.; Márton, A.; Szabados, M.; Varga, G.; Kónya, Z.; Kukovecz, Á.; Fülöp, F.; Pálinkó, I.; Ötvös, S. B. Exploiting a Silver-Bismuth Hybrid Material as Heterogeneous Noble Metal Catalyst for Decarboxylations and Decarboxylative Deuterations of Carboxylic Acids Under Batch and Continuous Flow Conditions. Green Chem. 2021, 23, 4685–4696.
- 54 Lee, C.-Y.; Ahn, S.-J.; Cheon, C.-H. Protodeboronation of ortho- and para-Phenol Boronic Acids and Application to ortho and meta Functionalization of Phenols Using Boronic Acids as Blocking and Directing Groups. J. Org. Chem. 2013, 78, 12154–12160.
- 55 Yan, B.; Zhou, Y.; Wu, J.; Ran, M.; Li, H.; Yao, Q. Catalyst-Free Reductive Hydrogenation or Deuteration of Aryl–Heteroatom Bonds Induced by Light. Org. Chem. Front. 2021, 8, 5244–5249.
- 56 Zhou, X.; Yu, T.; Dong, G. Site-Specific and Degree-Controlled Alkyl Deuteration via Cu-Catalyzed Redox-Neutral Deacylation. J. Am. Chem. Soc. 2022, 144, 9570–9575.
- 57 Ueno, K.; Hanada, A.; Yamaguchi, S.; Asami, T. Preparation of Multideuterated 5-Deoxystrigol for Use as an Internal Standard for Quantitative LC/MS. J. Labelled Compd. Rad. 2010, 53, 763–766.
- 58 De Feyter, H. M.; Behar, K. L.; Corbin, Z. A.; Fulbright, R. K.; Brown, P. B.; McIntyre, S.; Nixon, T. W.; Rothman, D. L.; de Graaf, R. A. Deuterium Metabolic Imaging (DMI) for MRI-Based 3D Mapping of Metabolism in vivo. Sci. Adv. 2018, 4, eaat7314.
- 59 Yao, J.; Dong, S.-C.; Tam, B. S. T.; Tang, C. W. Lifetime Enhancement and Degradation Study of Blue OLEDs Using Deuterated Materials. ACS Appl. Mater. Interfaces 2023, 15, 7255–7262.
- 60 Bae, H. J.; Kim, J. S.; Yakubovich, A.; Jeong, J.; Park, S.; Chwae, J.; Ishibe, S.; Jung, Y.; Rai, V. K.; Son, W. J.; Kim, S.; Choi, H.; Baik, M. H. Protecting Benzylic C-H Bonds by Deuteration Doubles the Operational Lifetime of Deep-Blue Ir-Phenylimidazole Dopants in Phosphorescent OLEDs. Adv. Opt. Mater. 2021, 9, 2100630.
- 61 Peng, X.; Yeh, C. H.; Wang, S. F.; Yan, J.; Gan, S.; Su, S. J.; Zhou, X.; Zhang, Y. X.; Chi, Y. Near-Infrared OLEDs Based on Functional Pyrazinyl Azolate Os(II) Phosphors and Deuteration. Adv. Opt. Mater. 2022, 10, 2201291.




