Synthesis of Anti-Pancreatic Cancer Natural Product Majusculamide D and Analogues Reveals a Preliminary Structure-Activity Relationships
Xiuhe Zhao
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorMengxue Lv
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorXiaonan Xi
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorYaxin Lu
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Liang Wang
College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yue Chen
College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXiuhe Zhao
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorMengxue Lv
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorXiaonan Xi
The State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy, Nankai University, Tianjin, 300350 China
Search for more papers by this authorYaxin Lu
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Liang Wang
College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yue Chen
College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
The total synthesis of majusculamide D (1) was achieved from commercially available materials. In addition, we synthesized eight analogues including three stereoisomers of majusculamide D that differ in the fatty acid chain. Six analogues including a simplified analogue 29 exhibited significant nanomolar-level IC50 values against Panc-1 cells in MTT assays. A preliminary SAR analysis indicated that the hydroxyl group at C10 and C2−C3 unsaturated double bond of majusculamide D were essential in maintaining the high activity against Panc-1 cells and the orientation of C40-Me and C42-Me groups was tolerable.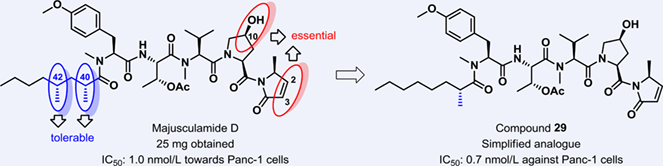
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300526-sup-0001-Supinfo.pdfPDF document, 8.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–17.
- 2 Peery, A. F.; Crockett, S. D.; Murphy, C. C.; Jensen, E. T.; Kim, H. P.; Egberg, M. D.; Lund, J. L.; Moon, A. M.; Pate, V.; Barnes, E. L.; Schlusser, C. L.; Baron, T. H.; Shaheen, N. J.; Sandler, R. S. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644.
- 3 Mizrahi, J. D.; Surana, R.; Valle, J. W.; Shroff, R. T. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- 4 Strobel, O.; Neoptolemos, J.; Jager, D.; Buchler, M. W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26.
- 5 Leao, T.; Castelao, G.; Korobeynikov, A.; Monroe, E. A.; Podell, S.; Glukhov, E.; Allen, E. E.; Gerwick, W. H.; Gerwick, L. Comparative genomics uncovers the prolific and distinctive metabolic potential of the cyanobacterial genus Moorea. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3198–3203.
- 6 Subramanian, B.; Nakeff, A.; Tenney, K.; Crews, P.; Gunatilaka, L.; Valeriote, F. A new paradigm for the development of anticancer agents from natural products. J. Exp. Ther. Oncol. 2006, 5, 195–204.
- 7 Newman, D. J.; Giddings, L. A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137.
- 8Do Rosário Martins, M.; Costa, M. Marine Cyanobacteria Compounds with Anticancer Properties: Implication of Apoptosis. In Handbook of Anticancer Drugs from Marine Origin, Ed.: Kim, S.-K., Springer International Publishing, Cham, 2015, pp. 621–647.
- 9 Moore, R. E.; Entzeroth, M. Majusculamide D and deoxymajusculamide D, two cytotoxins from Lyngbya majuscula. Phytochemistry 1988, 27, 3101–3103.
- 10 Caro-Diaz, E. J. E.; Valeriote, F. A.; Gerwick, W. H. Highly Convergent Total Synthesis and Assignment of Absolute Configuration of Majusculamide D, a Potent and Selective Cytotoxic Metabolite from Moorea sp. Org. Lett. 2019, 21, 793–796.
- 11 Koehn, F. E.; Longley, R. E.; Reed, J. K. Microcolins A and B, new immunosuppressive peptides from the blue-green alga Lyngbya majuscula. J. Nat. Prod. 1992, 55, 613–619.
- 12 Decicco, C. P.; Grover, P. Total Asymmetric Synthesis of the Potent Immunosuppressive Marine Natural Product Microcolin A. J. Org. Chem. 1996, 61, 3534–3541.
- 13 Mattern, R.-H.; Gunasekera, S.; McConnell, O. Synthetic studies of microcolin B. Tetrahedron 1996, 52, 425–434.
- 14 Andrus, M. B.; Li, W.; Keyes, R. F. Synthesis of Microcolin B, a Potent New Immunosuppressant Using an Efficient Mixed Imide Formation Reaction. J. Org. Chem. 1997, 62, 5542–5549.
- 15 Mandal, A. K.; Hines, J.; Kuramochi, K.; Crews, C. M. Developing microcolin A analogs as biological probes. Bioorg. Med. Chem. Lett. 2005, 15, 4043–4047.
- 16 Mattern, R.-H.; Gunasekera, S. P.; McConnell, O. J. Synthesis of microcolin analogs using trimethylsilylated lactams. Tetrahedron Lett. 1997, 38, 2197–2200.
- 17 Zhang, L.-H.; Longley, R. E. Induction of apoptosis in mouse thymocytes by microcolin A and its synthetic analog. Life Sci. 1999, 64, 1013–1028.
- 18 Koehn, F. E.; McConnell, O. J.; Longley, R. E.; Sennett, S. H.; Reed, J. K. Analogues of the marine immunosuppressant microcolin A: preparation and biological activity. J. Med. Chem. 1994, 37, 3181–3186.
- 19 Yu, H. B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P. C.; Jiao, B. H.; Gerwick, W. H. Cytotoxic Microcolin Lipopeptides from the Marine Cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619.
- 20 Rama Krishna, M. S.; Srinivasulu, B. Synthetic studies of Microcolin-B. Der Pharma Chem. 2012, 4, 1613–1618.
- 21 Iwasaki, A.; Ohno, O.; Sumimoto, S.; Ogawa, H.; Nguyen, K. A.; Suenaga, K. Jahanyne, an apoptosis-inducing lipopeptide from the marine cyanobacterium Lyngbya sp. Org. Lett. 2015, 17, 652–655.
- 22 Kallepu, S.; Kavitha, M.; Yeeravalli, R.; Manupati, K.; Jadav, S. S.; Das, A.; Mainkar, P. S.; Chandrasekhar, S. Total Synthesis of Desmethyl Jahanyne and Its Lipo-Tetrapeptide Conjugates Derived from Parent Skeleton as BCL-2-Mediated Apoptosis-Inducing Agents. ACS Omega 2018, 3, 63–75.
- 23 Siow, A.; Opiyo, G.; Kavianinia, I.; Li, F. F.; Furkert, D. P.; Harris, P. W. R.; Brimble, M. A. Total Synthesis of the Highly N-Methylated Acetylene-Containing Anticancer Peptide Jahanyne. Org. Lett. 2018, 20, 788–791.
- 24 Ye, B.; Jiang, P.; Zhang, T.; Ding, Y.; Sun, Y.; Hao, X.; Li, L.; Wang, L.; Chen, Y. Total Synthesis of the Highly N-Methylated Peptide Jahanyne. J. Org. Chem. 2018, 83, 6741–6747.
- 25 Iwasaki, A.; Fujimura, H.; Okamoto, S.; Kudo, T.; Hoshina, S.; Sumimoto, S.; Teruya, T.; Suenaga, K. Isolation of Jahanene and Jahanane, and Total Synthesis of the Jahanyne Family. J. Org. Chem. 2018, 83, 9592–9603.
- 26 Evans, D. A.; Ennis, M. D.; Mathre, D. J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982, 104, 1737–1739.
- 27 Evans, D. A.; Takacs, J. M. Enantioselective alkylation of chiral enolates. Tetrahedron Lett. 1980, 21, 4233–4236.
- 28 Carpino, L. A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 1993, 115, 4397–4398.
- 29
Carpino, L. A.; Imazumi, H.; El-Faham, A.; Ferrer, F. J.; Zhang, C.; Lee, Y.; Foxman, B. M.; Henklein, P.; Hanay, C.; Mügge, C.; Wenschuh, H.; Klose, J.; Beyermann, M.; Bienert, M. The Uronium/Guanidinium Peptide Coupling Reagents: Finally the True Uronium Salts. Angew. Chem. Int. Ed. 2002, 41, 441–445.
10.1002/1521-3773(20020201)41:3<441::AID-ANIE441>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar




