Modular Synthesis of Multi-substituted Cyclobutanones Enabled by Oxyallyl Cations†
Meng Wang
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Present address: Department of Cancer Biology, Dana-Farber Cancer Institute; Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA
Search for more papers by this authorZhonggui Wang
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Ping Lu
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorMeng Wang
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Present address: Department of Cancer Biology, Dana-Farber Cancer Institute; Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA
Search for more papers by this authorZhonggui Wang
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Ping Lu
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Stereoselective synthesis of multi-substituted cyclobutanes with different substituents is still a daunting challenge in organic synthesis. We report here a practical and facile approach to synthesizing all-trans 2,3,4-trisubstituted cyclobutanones from readily available dichlorocyclobutanones. The substitution reaction proceeds smoothly via oxyallyl cation intermediates under mild basic conditions. Further transformation to the synthesis of 1,2,3,4-tetrasubstituted cyclobutanes was also explored.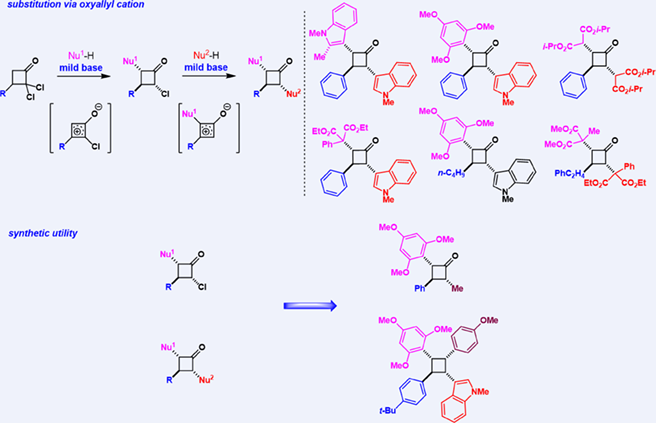
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300525-sup-0001-supinfo.pdfPDF document, 4.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Beniddir, M. A.; Evanno, L.; Joseph, D.; Skiredj, A.; Poupon, E. Emergence of Diversity and Stereochemical Outcomes in the Biosynthetic Pathways of Cyclobutane-Centered Marine Alkaloid Dimers. Nat. Prod. Rep. 2016, 33, 820–842; (b) Fan, Y.-Y.; Gao, X.-H.; Yue, J.-M. Attractive Natural Products with Strained Cyclopropane and/or Cyclobutane Ring Systems. Sci. China Chem. 2016, 59, 1126–1141.
- 2(a) Sadana, A. K.; Saini, R. K.; Billups, W. E. Cyclobutarenes and Related Compounds. Chem. Rev. 2003, 103, 1539–1602; (b) Xu, Y.; Conner, M. L.; Brown, M. K. Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2+2] Cycloadditions. Angew. Chem. Int. Ed. 2015, 54, 11918–11928; (c) Wang, M.; Lu, P. Catalytic Approaches to Assemble Cyclobutane Motifs in Natural Product Synthesis. Org. Chem. Front. 2018, 5, 254–259; (d) Li, J.; Gao, K.; Bian, M.; Ding, H. Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products. Org. Chem. Front. 2020, 7, 136–154.
- 3(a) Ohara, H.; Itoh, T.; Nakamura, M.; Nakamura, E. [2+2]-Cycloaddition Reaction of Styrene Derivatives using an Fe(III) Salt Catalyst. Chem. Lett. 2001, 30, 624–625; (b) Bauld, N. L.; Pabon, R. Cation Radical Catalyzed Olefin Cyclodimerization. J. Am. Chem. Soc. 1983, 105, 633–634; (c) Zou, Y.-Q.; Duan, S.-W.; Meng, X.-G.; Hu, X.-Q.; Gao, S.; Chen, J.-R.; Xiao, W.-J. Visible Light Induced Intermolecular [2+2]-Cycloaddition Reactions of 3-Ylideneoxindoles through Energy Transfer Pathway. Tetrahedron 2012, 68, 6914–6919; (d) Lei, T.; Zhou, C.; Huang, M. Y.; Zhao, L. M.; Yang, B.; Ye, C.; Xiao, H.; Meng, Q. Y.; Ramamurthy, V.; Tung, C.-H.; Wu, L.-Z. General and Efficient Intermolecular [2+2] Photodimerization of Chalcones and Cinnamic Acid Derivatives in Solution Through Visible-Light Catalysis. Angew. Chem. Int. Ed. 2017, 56, 15407–15410; (e) Pagire, S. K.; Hossain, A.; Traub, L.; Kerres, S.; Reiser, O. Photosensitised Regioselective [2+2]-Cycloaddition of Cinnamates and Related Alkenes. Chem. Commun. 2017, 53, 12072–12075; (f) Nguyen, L. V.; Jamison, T. F. Total Synthesis of (±)-Sceptrin. Org. Lett. 2020, 22, 6698–6702.
- 4 Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815.
- 5(a) Colomer, I.; Batchelor-Mcauley, C.; Odell, B.; Donohoe, T. J.; Compton, R. G. Hydrogen Bonding to Hexafluoroisopropanol Controls the Oxidative Strength of Hypervalent Iodine Reagents. J. Am. Chem. Soc. 2016, 138, 8855–8861; (b) Colomer, I.; Coura Barcelos, R.; Donohoe, T. J. Catalytic Hypervalent Iodine Promoters Lead to Styrene Dimerization and the Formation of Tri- and Tetrasubstituted Cyclobutanes. Angew. Chem. Int. Ed. 2016, 55, 4748–4752; (c) Yu, Y.; Fu, Y.; Zhong, F. Benign Catalysis with Iron: Facile Assembly of Cyclobutanes and Cyclohexenes via Intermolecular Radical Cation Cycloadditions. Green Chem. 2018, 20, 1743–1747; (d) Ischay, M. A.; Ament, M. S.; Yoon, T. P. Crossed Intermolecular [2 + 2] Cycloaddition of Styrenes by Visible Light Photocatalysis. Chem. Sci. 2012, 3, 2807–2811; (e) Riener, M.; Nicewicz, D. A. Synthesis of Cyclobutane Lignans via an Organic Single Electron Oxidant–Electron Relay System. Chem. Sci. 2013, 4, 2625–2629; (f) Das, S.; Zhu, C.; Demirbas, D.; Bill, E.; De, C. K.; List, B. Asymmetric Counteranion-Directed Photoredox Catalysis. Science 2023, 379, 494–499; (g) Ohmura, S.; Katagiri, K.; Kato, H.; Horibe, T.; Miyakawa, S.; Hasegawa, J.; Ishihara, K. J. Am. Chem. Soc. 2023, 145, 15054–15060.
- 6 Chen, J.; Zhou, Q.; Fang, H.; Lu, P. Dancing On Ropes–Enantioselective Functionalization of Preformed Four-Membered Carbocycles. Chin. J. Chem. 2022, 40, 1346–1358.
- 7(a) Gutekunst, W. R.; Baran, P. S. Total Synthesis and Structural Revision of the Piperarborenines via Sequential Cyclobutane C–H Arylation. J. Am. Chem. Soc. 2011, 133, 19076–19079; (b) Gutekunst, W. R.; Baran, P. S. Applications of C–H Functionalization Logic to Cyclobutane Synthesis. J. Org. Chem. 2014, 79, 2430–2452.
- 8(a) Harmata, M. The (4+3)-Cycloaddition Reaction: Simple Allylic Cations as Dienophiles. Chem. Commun. 2010, 46, 8886–8903; (b) Wu, J.; Li, H. (3+2)-Cycloaddition Reactions of Oxyallyl Cations. Synthesis 2015, 47, 22–33.
- 9(a) Tang, Q.; Chen, X.; Tiwari, B.; Chi, Y. R. Addition of Indoles to Oxyallyl Cations for Facile Access to α-Indole Carbonyl Compounds. Org. Lett. 2012, 14, 1922–1925; (b) Vander Wal, M. N.; Dilger, A. K.; MacMillan, D. W. C. Development of A Generic Activation Mode: Nucleophilic α-Substitution of Ketones via Oxy-allyl Cations. Chem. Sci. 2013, 4, 3075–3079; (c) Liu, C.; Oblak, E. Z.; Vander Wal, M. N.; Dilger, A. K.; Almstead, D. K.; Macmillan, D. W. C. Oxy-Allyl Cation Catalysis: An Enantioselective Electrophilic Activation Mode. J. Am. Chem. Soc. 2016, 138, 2134–2137.
- 10 Depres, J.-P.; Delair, P.; Poisson, J.-F.; Kanazawa, A.; Greene, A. E. Diverse Natural Products from Dichlorocyclobutanones: An Evolutionary Tale. Acc. Chem. Res. 2016, 49, 252–261.
- 11(a) Hassner, A.; Dillon, J. L.; Krepski, L. R.; Onan, K. D. Effect of Conformation and Stereochemistry in Substitution Reactions of Fused Chlorocyclobutanones: Oxyallyl Cation Intermediates. Tetrahedron Lett. 1983, 24, 1135–1138; (b) Hassner, A.; Naidorf-Meir, S. Synthetic Methods. α-Substituted Cyclobutanones as Protecting Groups for Carboxylic Acids. J. Org. Chem. 1989, 54, 4954–4957; (c) Hassner, A.; Naidorf-Meir, S.; Frimer, A. A. Cine Substitution in 2-Oxabicyclo[4.2.0]octanones and Subsequent Unusual Rearrangements. J. Org. Chem. 1996, 61, 4051–4058.
- 12Deposition Numbers 2291119 (1l), 2291120 (4a), 2291121 (5g), 2291122 (5i), and 2291123 (2a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 13 Depres, J.-P.; Greene, A. E. Transformations of α,α-Dichlorocyclopentanones Obtained by Three-Carbon Annelation. J. Org. Chem. 1980, 45, 2036–2037.
- 14For recent examples of ring-opening reaction, see: (a) Chen, P.-h.; Billett, B. A.; Tsukamoto, T.; Dong, G. “Cut and Sew” Transformations via Transition-Metal-Catalyzed Carbon–Carbon Bond Activation. ACS Catal. 2017, 7, 1340–1360; (b) Xue, Y.; Dong, G. Deconstructive Synthesis of Bridged and Fused Rings via Transition-Metal-Catalyzed “Cut-and-Sew” Reactions of Benzocyclobutenones and Cyclobutanones. Acc. Chem. Res. 2022, 55, 2341–2354; (c) Li, R.; Li, B.; Zhang, H.; Ju, C.-W.; Qin, Y.; Xue, X.-S.; Zhao, D. A Ring Expansion Strategy towards Diverse Azaheterocycles. Nat. Chem. 2021, 13, 1006–1016; (d) Li, R.; Shi, X.; Zhao, D. Palladium-Catalyzed Skeletal Reorganization of Cyclobutanones Invoving Successive C—C Bond/C—H Bond Cleavage. Chin. J. Chem. 2023, 41, 1679–1683.




