Cooperative NHC/Photoredox Catalysis: Three-Component Reaction of Aroyl Fluorides, Styrenes and Oxalates
Zheng Lin
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorGuanghui Lv
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Department of Pharmacy, Hubei Provincial Clinical Research Center for Umbilical Cord Blood Hematopoietic Stem Cells, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000 China
Search for more papers by this authorJianghong Liu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJiangyan Tang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorBinsong Mu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJian Chen
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorTianle Huang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorCorresponding Author
Zhongzhen Yang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yong Wu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZheng Lin
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorGuanghui Lv
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Department of Pharmacy, Hubei Provincial Clinical Research Center for Umbilical Cord Blood Hematopoietic Stem Cells, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000 China
Search for more papers by this authorJianghong Liu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJiangyan Tang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorBinsong Mu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJian Chen
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorTianle Huang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
Search for more papers by this authorCorresponding Author
Zhongzhen Yang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yong Wu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, 610041 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Cooperative NHC/photoredox catalysis has emerged as an important research field in recent years. Herein, this article describes the use of cesium salt derivatives of tert-alcohols as alkyl radical precursors in a three-component reaction with styrene and aromatic acyl fluorides to synthesize α-arylalkyl aryl ketones. The aroyl fluoride reacts with the NHC catalyst, leading to the formation of an acyl azolium ion. This acyl azolium ion can then be reduced by the photoredox catalyst, generating the corresponding ketyl radical anion. The C-radical generated from oxalate oxidation undergoes an addition reaction with the styrene derivative, followed by cross-coupling of the addition radical with the ketone radical, which is fragmented by NHC to give the target ketone.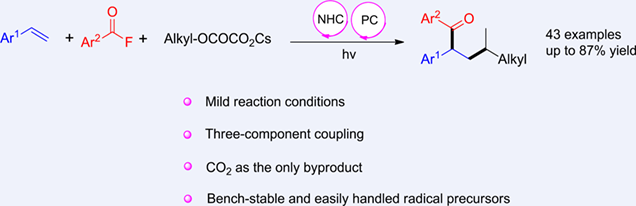
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300524-sup-0001-supinfo.pdfPDF document, 5.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 McDaniel, R.; Thamchaipenet, A.; Gustafsson, C.; Fu, H.; Betlach, M.; Betlach, M.; Ashley, G. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel "unnatural" natural products. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 1846–1851.
- 2 Walter, M. W. Structure-based design of agrochemicals. Nat. Prod. Rep. 2002, 19, 278–291.
- 3 Dieter, R. K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236.
- 4 Rueping, M.; Nachtsheim, B. J. A review of new developments in the Friedel-Crafts alkylation - From green chemistry to asymmetric catalysis. Beilstein. J. Org. Chem 2010, 6, 6.
- 5 Heravi, M. M.; Zadsirjan, V.; Saedi, P.; Momeni, T. Applications of Friedel-Crafts reactions in total synthesis of natural products. RSC Adv. 2018, 8, 40061–40163.
- 6 Milligan, J. A.; Phelan, J. P.; Badir, S. O.; Molander, G. A. Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem. Int. Ed. 2019, 58, 6152–6163.
- 7 Joe, C. L.; Doyle, A. G. Direct Acylation of C(sp3)-H Bonds Enabled by Nickel and Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 4040–4043.
- 8 Buchspies, J.; Szostak, M. Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts 2019, 9, 53.
- 9 Takise, R.; Muto, K.; Yamaguchi, J. Cross-coupling of aromatic esters and amides. Chem. Soc. Rev. 2017, 46, 5864–5888.
- 10 Guin, J.; De Sarkar, S.; Grimme, S.; Studer, A. Biomimetic carbene-catalyzed oxidations of aldehydes using TEMPO. Angew. Chem. Int. Ed. 2008, 47, 8727–8730.
- 11 Pareek, M.; Reddi, Y.; Sunoj, R. B. Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: then and now. Chem. Sci.2021, 12, 7973–7992.
- 12 Chalotra, N.; Sultan, S.; Shah, B. A. Recent Advances in Photoredox Methods for Ketone Synthesis. Asian J. Org. Chem 2020, 9, 863–881.
- 13 Liu, J.; Xing, X. N.; Huang, J. H.; Lu, L. Q.; Xiao, W. J. Light opens a new window for N-heterocyclic carbene catalysis. Chem. Sci. 2020, 11, 10605–10613.
- 14 Chen, K. Q.; Sheng, H.; Liu, Q.; Shao, P. L.; Chen, X. Y. N-Heterocyclic carbene-catalyzed radical reactions. Sci. China-Chem. 2021, 64, 7–16.
- 15 Liu, K.; Schwenzer, M.; Studer, A. Radical NHC Catalysis. ACS Catal. 2022, 12, 11984–11999.
- 16 Liu, M. S.; Min, L.; Chen, B. H.; Shu, W. Dual Catalysis Relay: Coupling of Aldehydes and Alkenes Enabled by Visible-Light and NHC-Catalyzed Cross-Double C-H Functionalizations. ACS Catal. 2021, 11, 9715–9721.
- 17 Liu, M. S.; Shu, W. Catalytic, Metal-Free Amide Synthesis from Aldehydes and Imines Enabled by a Dual-Catalyzed Umpolung Strategy under Redox-Neutral Conditions. ACS Catal. 2020, 10, 12960–12966.
- 18 Meng, Q. Y.; Doben, N.; Studer, A. Cooperative NHC and Photoredox Catalysis for the Synthesis of beta-Trifluoromethylated Alkyl Aryl Ketones. Angew. Chem. Int. Ed. 2020, 59, 19956–19960.
- 19 Zhang, B.; Qi, J. Q.; Liu, Y. H.; Li, Z. P.; Wang, J. Visible-Light-Driven Bisfunctionalization of Unactivated Olefins via the Merger of Proton-Coupled Electron Transfer and Carbene Catalysis. Org. Lett. 2022, 24, 279–283.
- 20 Du, H. W.; Liu, M. S.; Shu, W. Synthesis of beta-Thiolated-alpha-arylated Ketones Enabled by Photoredox and N-Heterocyclic Carbene- Catalyzed Radical Relay of Alkenes with Disulfides and Aldehydes. Org. Lett. 2022, 24, 5519–5524.
- 21 Zhang, B.; Wang, J. Acyldifluoromethylation Enabled by NHC-Photoredox Cocatalysis. Org. Lett. 2022, 24, 3721–3725.
- 22 Doben, N.; Reimler, J.; Studer, A. Cooperative NHC/Photoredox Catalysis: Three Component Radical Coupling of Aroyl Fluorides, Styrenes and Alcohols. Adv. Synth. Catal. 2022, 364, 3348–3353.
- 23 Ren, S. C.; Yang, X.; Mondal, B.; Mou, C. L.; Tian, W. Y.; Jin, Z. C.; Chi, Y. R. Carbene and photocatalyst-catalyzed decarboxylative radical coupling of carboxylic acids and acyl imidazoles to form ketones. Nat. Commun. 2022, 13, 2846.
- 24 Tan, C. Y.; Kim, M.; Hong, S. W. Photoinduced Electron Transfer from Xanthates to Acyl Azoliums: Divergent Ketone Synthesis via N-Heterocyclic Carbene Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202306191
- 25 Nawrat, C. C.; Jamison, C. R.; Slutskyy, Y.; MacMillan, D. W. C.; Overman, L. E. Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling. J. Am. Chem. Soc. 2015, 137, 11270–11273.




