Recent Advances in Pd-Catalyzed Reactions Involving the “On-Water” Mechanism†
Corresponding Author
Dong Wei
State Key Laboratory of Systems Medicine for Cancer, Shanghai Cancer Institute, Shanghai Key Laboratory of Systems Regulation and Clinical Translation for Cancer, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guo-Qiang Lin
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong Wei
State Key Laboratory of Systems Medicine for Cancer, Shanghai Cancer Institute, Shanghai Key Laboratory of Systems Regulation and Clinical Translation for Cancer, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guo-Qiang Lin
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Memory of Professor Xiyan Lu.
Abstract
Comprehensive Summary
As the chemical industry grapples with the need for more eco-friendly practices, the use of water as a reaction medium is gaining attraction in organic transformations. This mini-review delves into Pd-catalyzed reactions that utilize the "on-water" mechanism, spanning from 2019 to late 2023. These reactions are neatly categorized into several types: (A) Catalytic C—H activations, (B) Mizoroki- Heck-type reactions, (C) Suzuki-Miyaura reactions, and (D) Cyclization reactions. By showcasing the potential of water as a sustainable reaction medium for organic transformations, these reactions leave no doubt about the importance of embracing eco-friendly practices in the chemical industry.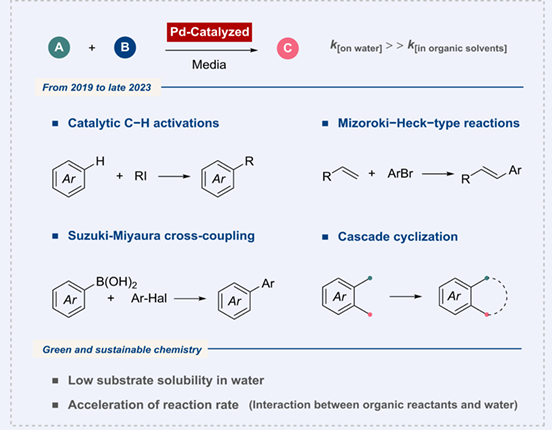
Key Scientists
In 1980, a seminal work by Breslow et al. showed an acceleration of reaction rate in the Diels-Alder reaction. Sharpless and co-workers noted a significant increase in the rate of the [2σ+2σ+2π] cycloaddition of quadricyclane and dimethyl azodicarboxylate (DMAD) when the reaction was conducted in water, as opposed to when it was carried out in organic solvents. The term "on-water" was then coined to describe this phenomenon. This strategy was further expanded to transition-metal catalyzed transformations by Ackermann in 2011. Later, Varma and Leazer disclosed a Pd-catalyzed Mizoroki-Heck type arylation of alkenes with diaryliodonium salts “on-water”. The enantioselective version of "on-water" process was not realized until 2014 by the Zhou group. Later on, the Schaub group described a Pd-catalyzed Suzuki–Miyaura coupling reaction of electron-poor aryl chlorides with water, using only 50 ppm of catalyst loading. Very recently, Liu and Lin extended the "on-water" strategy to Pd-catalyzed double Mizoroki-Heck reactions. This mini-review has focused on Pd-catalyzed reactions involving the “on-water” mechanism.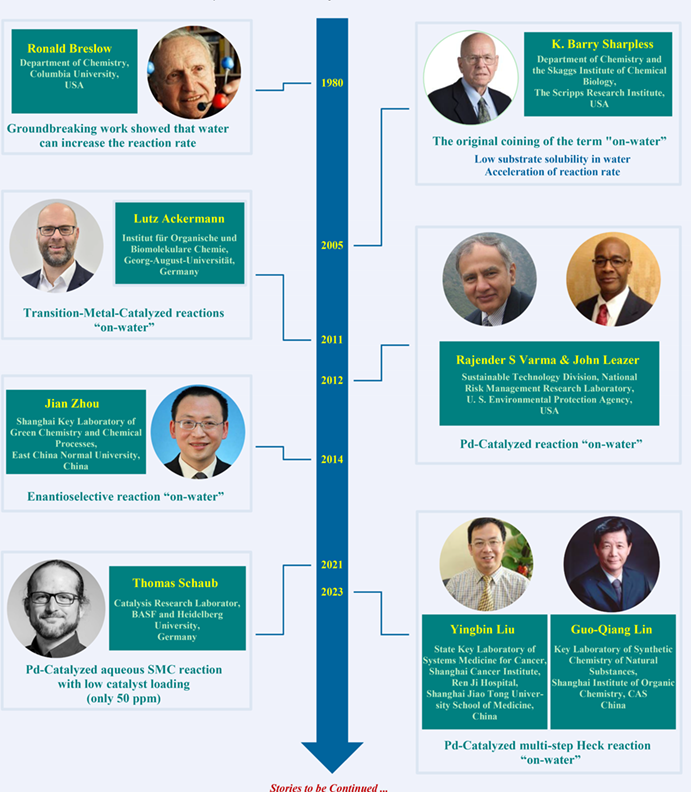
References
- 1 Polshettiwar, V.; Varma, R. S. Aqueous microwave chemistry: a clean and green synthetic tool for rapid drug discovery. Chem. Soc. Rev. 2008, 37, 1546–1557.
- 2 Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312.
- 3 Simon. M. O.; Li, C. J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427.
- 4 Breynaert, E.; Houlleberghs, M.; Radhakrishnan, S.; Grübel, G.; Taulelle, F.; Martens, J. A. Water as a tuneable solvent: a perspective. Chem. Soc. Rev. 2020, 49, 2557–2569.
- 5 Sahoo, T.; Panda, J.; Sahu, J.; Sarangi, D.; Sahoo, S. K.; Nanda, B. B.; Sahu, R. Green Solvent: Green Shadow on Chemical Synthesis. Curr. Org. Synth. 2020, 17, 426–439.
- 6 Cortes-Clerget, M.; Yu, J.; Kincaid, J. R. A.; Walde, P.; Gallou, F.; Lipshutz, B. H. Water as the reaction medium in organic chemistry: from our worst enemy to our best friend. Chem. Sci. 2021, 12, 4237–4266.
- 7 Rideout, D. C.; Breslow, R. Hydrophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817.
- 8 Narayan, S.; Muldoon, J.; Finn, M. G.; Fokin, V. V.; Kolb, H. C.; Sharpless, K. B. "On water": Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279.
- 9 Minakata, S.; Komatsu, M. Organic Reactions on Silica in Water. Chem. Rev. 2009, 109, 711–724.
- 10 Butler, R. N.; Coyne, A. G. Water: Nature's Reaction Enforcer-Comparative Effects for Organic Synthesis “In-Water” and “On-Water”. Chem. Rev. 2010, 110, 6302–6337.
- 11 Zheng, Y.; Zhang, J. Catalysis in the Oil Droplet/Water Interface for Aromatic Claisen Rearrangement. J. Phys. Chem. A 2010, 114, 4325–4333.
- 12 Gawande, M. B.; Bonifacio, V. D. B.; Luque, R.; Branco, P. S.; Varma, R. S. Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551.
- 13 Yuan, H.; Zheng, Y.; Fang, Z.; Bi, X.; Zhang, J. Mechanistic understanding of domino cyclization between gem-dialkylthio vinylallenes and benzylamine towards eco-nomic synthesis: a computational study. Green Chem. 2014, 16, 2653–2663.
- 14 Lam, H. C.; Pepper, H. P.; Sumby, C. J.; George, J. H. Biomimetic Total Synthesis of (+/-)-Verrubenzospirolactone. Angew. Chem. Int. Ed. 2017, 56, 8532–8535.
- 15 Harry, N. A.; Radhika, S.; Neetha, M.; Anilkumar, G. Recent Advances and Prospects of Organic Reactions “On Water”. ChemistrySelect 2019, 4, 12337–12355.
- 16 Cioc, R. C.; Smak, T. J.; Crockatt, M.; van der Waal, J. C.; Bruijnincx, P. C. A. Furoic acid and derivatives as atypical dienes in Diels-Alder reactions. Green Chem. 2021, 23, 5503–5510.
- 17 Kitanosono, T.; Kobayashi, S. Synthetic Organic "Aquachemistry" that Relies on Neither Cosolvents nor Surfactants. ACS Cent. Sci. 2021, 7, 739–747.
- 18 Kitanosono, T.; Kobayashi, S. Reactions in Water Involving the "On-Water" Mechanism. Chem.-Eur. J. 2020, 26, 9408–9429.
- 19 Shi, F. Q.; Li, X.; Xia, Y.; Zhang, L.; Yu, Z. X. DFT Study of the Mechanisms of In Water Au(I)-Catalyzed Tandem [3,3]-Rearrangement /Nazarov Reaction/[1,2]-Hydrogen Shift of Enynyl Acetates: A Proton-Transport Catalysis Strategy in the Water-Catalyzed [1,2]-Hydrogen Shift. J. Am. Chem. Soc. 2007, 129, 15503–15512.
- 20 Liang, Y.; Zhou, H.; Yu, Z. X. Why Is Copper(I) Complex More Competent Than Dirhodium(II) Complex in Catalytic Asymmetric O-H Insertion Reactions? A Computational Study of the Metal Carbenoid O-H Insertion into Water. J. Am. Chem. Soc. 2009, 131, 17783–17785.
- 21 Sengoden, M.; Punniyamurthy, T. “On Water”: Efficient Iron-Catalyzed Cycloaddition of Aziridines with Heterocumulenes. Angew. Chem. Int. Ed., 2013, 52, 572–575.
- 22 Scalambra, F.; Lorenzo-Luis, P.; Rios, I. d. l.; Romeros, A. New achievements on C-C bond formation in water catalyzed by metal complexes. Coord. Chem. Rev. 2021, 443, 213997.
- 23 Peng, K.; Dong, Z. B. Recent Advances in Cross-Dehydrogenative Couplings (CDC) of C-H Bond in Aqueous Media. Adv. Synth. Catal. 2021, 363, 1185–1201.
- 24 Dhawa, U.; Kaplaneris, N.; Ackermann, L. Green strategies for transition metal-catalyzed C–H activation in molecular syntheses. Org. Chem. Front. 2021, 8, 4886–4913.
- 25 Crisp, G. T. Variations on a theme - recent developments on the mechanism of the Heck reaction and their implications for synthesis. Chem. Soc. Rev. 1998, 27, 427–436.
- 26 Beletskaya, I. P.; Cheprakov, A. V. The heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066.
- 27 Xie, J. Q.; Liang, R. X.; Jia, Y. X. Recent Advances of Catalytic Enantioselective Heck Reactions and Reductive-Heck Reactions. Chin. J. Chem. 2021, 39, 710–728.
- 28 Wei, D.; Lin, G. Q. Functionalization of C,C-palladacycles: application in the synthesis of functional molecules. Sci. China Chem. 2023, 66, 2721–2733.
- 29 Shaughnessy, K. H.; DeVasher, R. B. Palladium-catalyzed cross-coupling in aqueous media: Recent progress and current applications. Curr. Org. Chem. 2005, 9, 585–604.
- 30 Yousaf, M.; Zahoor, A. F.; Akhtar, R.; Ahmad, M.; Naheed, S. Development of green methodologies for Heck, Chan-Lam, Stille and Suzuki cross-coupling reactions. Mol. Divers. 2020, 24, 821–839.
- 31 Cera, G.; Maestri, G. Palladium/Brønsted Acid Catalysis for Hydrofunctionalizations of Alkynes: From Tsuji-Trost Allylations to Stereoselective Methodologies. ChemCatChem 2022, 14, e202200295.
- 32 Ansari, T. N.; Gallou, F.; Handa, S. Palladium-catalyzed micellar cross-couplings: An outlook. Coord. Chem. Rev. 2023, 488, 215158.
- 33 Butler, R. N.; Coyne, A. G. Water: Nature's Reaction Enforcers-Comparative Effects for Organic Synthesis “In-Water” and “On-Water”. Chem. Rev. 2010, 110, 6302–6337.
- 34 Dong, D. Q.; Yang, H.; Zhou, M. Y.; Wei, Z. H.; Wu, P.; Wang, Z. L. Recent advances in palladium-catalyzed reactions in water. Curr. Opin. Green. Sust. 2023, 40, 100778
- 35 Welin, E. R.; Ngamnithiporn, A.; Klatte, M.; Lapointe, G.; Pototschnig, G. M.; McDermott, M. S. J.; Conklin, D.; Gilmore, C. D.; Tadross, P. M.; Haley, C. K.; Negoro, K.; Glibstrup, E.; Grünanger, C. U.; Allan, K. M.; Virgil, S. C.; Slamon, D. J.; Stoltz, B. M. Concise total syntheses of (–)-jorunnamycin A and (–)-jorumycin enabled by asymmetric catalysis. Science 2019, 363, 270.
- 36 Çapcı, A.; Lorion, M. M.; Wang, H.; Simon, N.; Leidenberger, M.; Borges Silva, M. C.; Moreira, D. R. M.; Zhu, Y.; Meng, Y.; Chen, J. Y.; Lee, Y. M.; Friedrich, O.; Kappes, B.; Wang, J.; Ackermann, L.; Tsogoeva, S. B. Artemisinin-(Iso)quinoline Hybrids by C−H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. 2019, 58, 13066–13079.
- 37 Elguero, J. Comprehensive Heterocyclic Chemistry, Eds.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Pergamon, Oxford, UK, 1996, Vol. 3, pp. 1–75.
- 38 Gambouz, K.; Abbouchi, A. E.; Nassiri, S.; Suzenet, F.; Bousmina, M.; Akssira, M.; Guillaumet, G.; Kazzouli, S. E. “On Water” Palladium Catalyzed Direct Arylation of 1H-Indazole and 1H-7-Azaindazole. Molecules 2020, 25, 2820.
- 39 Verma, A.; Dolui, P.; Hazra, S.; Elias, A. J. Directing group enabled ‘On-Water’ C-H bond functionalization of ferrocene derivatives. J. Organomet. Chem. 2022, 964, 122303.
- 40 Verma, A.; Elias, A. J. C-H Bond Functionalization of Aryl Acids and Amines by ‘On-water’ Reaction: Bi-dentate Directing Group Enabled Synthesis of Biaryl and m-Teraryl Carboxamides. Chem. Asian J. 2023, 18, e202300191.
- 41 Christoffel, F.; Ward, T. R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2018, 148, 489–511.
- 42 Kaur, N.; Kaur, G.; Bhalla, A.; Dhau, J. S.; Chaudhary, G. R. Metallosurfactant based Pd–Ni alloy nanoparticles as a proficient catalyst in the Mizoroki Heck coupling reaction. Green Chem. 2018, 20, 1506–1514.
- 43 Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B. H. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki–Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382.
- 44 Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374.
- 45 Patil, S. P.; Jadhav. S. N.; Rode, C. V.; Shejwal, R. V.; Kumbhar, A. S. Bio-surfactant: a green and environmentally benign reaction medium for ligand-free Pd-catalyzed Mizoroki-Heck cross-coupling reaction in water. Transit. Met. Chem. 2020, 45, 403–411.
- 46 Tezuka, Y.; Honda, K.; Banskota, A. H.; Thet, M. M.; Kadota, S. Kinmoonosides A−C, Three New Cytotoxic Saponins from the Fruits of Acacia concinna, a Medicinal Plant Collected in Myanmar. J. Nat. Prod. 2000, 63, 1658–1664.
- 47 Wei, D.; Lu, H. Y.; Miao, H. Z.; Feng, C. G.; Lin, G. Q.; Liu, Y. Pd-catalyzed intermolecular consecutive double Heck reaction “on water” under air: facile synthesis of substituted indenes. RSC Adv. 2023, 13, 19312–19316.
- 48 Abel-Snape, X.; Wycich, G.; Lautens, M. Synthesis of Indenes and Benzofulvenes via a Palladium-Catalyzed Three-Component Reaction. ACS Catal. 2022, 12, 3291–3301.
- 49 Suzuki, A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737.
- 50 Molnár, Á. Efficient, Selective, and Recyclable Palladium Catalysts in Carbon-Carbon Coupling Reactions. Chem. Rev. 2011, 111, 2251–2320.
- 51 Thunga, S.; Poshala, S.; Anugu, N.; Konakanchi, R.; Vanaparthi, S.; Kokatla, H. P. An efficient Pd(II)-(2-aminonicotinaldehyde) complex as complementary catalyst for the Suzuki-Miyaura coupling in water. Tetrahedron Lett. 2019, 60, 2046–2048.
- 52 Wang, Y.; Liu, Y.; Zhang, W. Q.; Sun, H.; Zhang, K.; Jian, Y.; Gu, Q.; Zhang, G.; Li, J.; Gao, Z. Sustainable Ligand-Free, Palladium-Catalyzed Suzuki-Miyaura Reactions in Water: Insights into the Role of Base. ChemSusChem 2019, 12, 5265–5273.
- 53 Galushko, A. S.; Prima, D. O.; Burykina, J. V.; Ananikov, V. P. Comparative study of aryl halides in Pd-mediated reactions: key factors beyond the oxidative addition step. Inorg. Chem. Front. 2021, 8, 620–635.
- 54 Orecchia, P.; Petkova, D. S.; Goetz, R.; Rominger, F.; Hashmi, A. S. K.; Schaub, T. Pd-Catalysed Suzuki-Miyaura cross-coupling of aryl chlorides at low catalyst loadings in water for the synthesis of industrially important fungicides. Green Chem. 2021, 23, 8169–8180.
- 55 Wan, Y.; Song, F.; Ye, T.; Li, G.; Liu, D.; Lei, Y. Carbonylative Suzuki coupling and alkoxycarbonylation of aryl halides using palladium supported on phosphorus-doped porous organic polymer as an active and robust catalyst. Appl. Organomet. Chem. 2019, 33, e4714.
- 56 Lei, Y.; Chena, Z.; Li, G. Palladium/phosphorus-functionalized porous organic polymer with tunable surface wettability for water-mediated Suzuki–Miyaura coupling reaction. RSC Adv. 2019, 9, 36600–36607.
- 57 Zhang, Y.; Tortorella, M. D.; Liao, J.; Qin, X.; Chen, T.; Luo, J.; Guan, J.; Talley, J. J.; Tu, Z. Synthesis and Evaluation of Novel Erlotinib-NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med. Chem. Lett. 2015, 6, 1086–1090.
- 58 Jafari, E.; Khajouei, M. R.; Hassanzadeh, F.; Hakimelahi, G. H.; Khodarahmi, G. A. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sc. 2016, 11, 1–14.
- 59 Yuan, S.; Yu, B.; Liu, H. M. “On-Water” Palladium-Catalyzed Tandem Cyclization Reaction for the Synthesis of Biologically Relevant 4-Arylquinazolines. Chem. Eur. J. 2019, 25, 13109–13113.
- 60 Hoegenauer, K.; Soldermann, N.; Stauffer, F.; Furet, P.; Graveleau, N.; Smith, A. B.; Hebach, C.; Hollingworth, G. J.; Lewis, I.; Gutmann, S.; Rummel, G.; Knapp, M.; Wolf, R. M.; Blanz, J.; Feifel, R.; Burkhart, C.; Zecri, F. Discovery and Pharmacological Characterization of Novel Quinazoline-Based PI3K Delta-Selective Inhibitors. ACS Med. Chem. Lett. 2016, 7, 762–767.




