Palladium(II)-Catalyzed Markovnikov Hydroalkynylation of Unactivated Terminal Alkenes†
Chuanqi Hou
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Pinhong Chen
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guosheng Liu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Chang-Kung Chuang Institute, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]Search for more papers by this authorChuanqi Hou
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Pinhong Chen
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guosheng Liu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Chang-Kung Chuang Institute, East China Normal University, Shanghai, 200062 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Alkynes are versatile synthons in organic synthesis, as well as important structural moieties in bioactive molecules. Recently, transition metal-catalyzed hydroalkynylation of alkenes has been developed with reactive alkenes and alkenes bearing directing groups. However, the regioselective hydroalkynylation of simple alkenes is still challenging. Herein, we have developed a palladium-catalyzed Markovnikov hydroalkynylation of unactivated terminal alkenes, which provides an efficient approach for the synthesis of branched alkynyl compounds under mild conditions. This reaction features excellent functional group tolerance, good reaction yields and excellent regioselectivity. Moreover, the asymmetric hydroalkynylation reaction has also been achieved with moderate enantioselectivity by introducing a sterically bulky chiral Pyox ligand.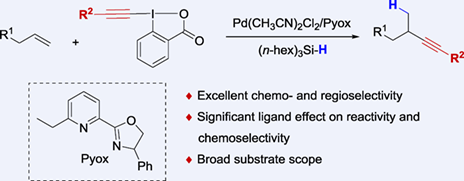
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300472-sup-0001-supinfo.pdfPDF document, 2.4 MB |
Appendix S1: Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Zeni, G.; Laroc, R. C. Synthesis of Heterocycles via Palladium π-Olefin and π-Alkyne Chemistry. Chem. Rev. 2004, 104, 2285–2309; (b) Meldal, M.; Tornøe, C. W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015.
- 2(a) Brooks, C. D. W.; Summers, J. B. Modulators of Leukotriene Biosynthesis and Receptor Activation. J. Med. Chem. 1996, 39, 2629–2654; (b) Krasowski, M. D.; Nishikawa, K.; Nikolaeva, N.; Lin, A.; Harrison, N. L. Methionine 286 in transmembrane domain 3 of the GABAA receptor β subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology 2001, 41, 952–964; (c) Xie, Y.; Tao, W.; Morrison, H.; Chiu, R.; Jona, J.; Fang, J.; Cauchon, N. Quantitative determination of solid-state forms of a pharmaceutical development compound in drug substance and tablets. Int. J. Pharm. 2008, 362, 29–36.
- 3For reviews, see: (a) Wang, Z.-X.; Bai, X.-Y.; Li, B.-J. Metal-Catalyzed Substrate-Directed Enantioselective Functionalization of Unactivated Alkenes. Chin. J. Chem. 2019, 37, 1174–1180; (b) Huang, J.; Chen, Z.-M. The Alkynylative Difunctionalization of Alkenes. Chem. Eur. J. 2022, e202201519; (c) He, Y.; Chen, J.; Jian, X.; Zhu, S. Enantioselective NiH-Catalyzed Reductive Hydrofunctionalization of Alkenes. Chin. J. Chem. 2021, 40, 651–661; (d) Zhao, W.; Lu, H.-X.; Zhang, W.-W.; Li, B.-J. Coordination Assistance: A Powerful Strategy for Metal-Catalyzed Regio- and Enantioselective Hydroalkynylation of Internal Alkenes. Acc. Chem. Res. 2023, 56, 308–321.
- 4(a) Chen, L.; Li, C. J. The first palladium-catalyzed 1,4-addition of terminal alkynes to conjugated enones. Chem. Commun. 2004, 2362–2364; (b) Villarino, L.; Fandino, R. G.; Lopez, F.; Mascarenas, J. L. Palladium-Catalyzed Conjugate Addition of Terminal Alkynes to Enones. Org. Lett. 2012, 14, 2996–2999; (c) Chen, Z. M.; Nervig, C. S.; DeLuca, R. J.; Sigman, M. S. Palladium-Catalyzed Enantioselective Redox-Relay Heck Alkynylation of Alkenols to Access Propargylic Stereocenters. Angew. Chem. Int. Ed. 2017, 56, 6651–6654; (d) Bai, Z.; Bai, Z.; Song, F.; Wang, H.; Chen, G.; He, G. Palladium-Catalyzed Amide-Directed Hydrocarbo- functionalization of 3-Alkenamides with Alkynes. ACS Catal. 2020, 10, 933–940; (e) Dian, L.; Marek, I. Pd-Catalyzed Enantioselective Hydroalkynylation of Cyclopropenes. ACS Catal. 2020, 10, 1289–1293; (f) Shukla, R. K.; Chaturvedi, A. K.; Pal, S.; Volla, C. M. R. Catalytic, Regioselective Hydrocarbofunctionalization of Unactivated Alkenes Triggered by trans-Acetoxypalladation of Alkynes. Org. Lett. 2021, 23, 1440–1444; (g) Simlandy, A. K.; Alturaifi, T. M.; Nguyen, J. M.; Oxtoby, L. J.; Wong, Q. N.; Chen, J. S.; Liu, P.; Engle, K. M. Enantioselective Hydroalkenylation and Hydroalkynylation of Alkenes Enabled by a Transient Directing Group: Catalyst Generality through Rigidification. Angew. Chem. Int. Ed. 2023, e20230401.
- 5(a) Shirakura, M.; Suginome, M. Nickel-Catalyzed Addition of C-H Bonds of Terminal Alkynes to 1,3-Dienes and Styrenes. J. Am. Chem. Soc. 2008, 130, 5410–5411; (b) Larionov, O. V.; Corey, E. J. Ni(II)-Catalyzed Enantioselective Conjugate Addition of Acetylenes to α,β-Enones. Org. Lett. 2009, 12, 300–302; (c) Masamichi, S.; Suginome, M. Nickel-Catalyzed Regioselective Hydroalkynylation of Styrenes: Improved Catalyst System, Reaction Scope, and Mechanism. Org. Lett. 2009, 11, 523–526; (d) Shirakura, M.; Suginome, M. Nickel-Catalyzed Asymmetric Addition of Alkyne C-H Bonds across 1,3-Dienes Using Taddol-Based Chiral Phosphoramidite Ligands. Angew. Chem. In. Ed. 2010, 49, 3827–3829.
- 6(a) Lerum, R. V.; Chisholm, J. D. Rhodium-catalyzed 1,4-addition of terminal alkynes to vinyl ketones. Tetrahedron Lett. 2004, 45, 6591–6594; (b) Nishimura, T.; Guo, X. X.; Hayashi, T. Rhodium-catalyzed asymmetric addition of terminal alkynes to diarylphosphinylallenes. Chem. Asian J. 2008, 3, 1505–1510; (c) Sawano, T.; Hashizume, M.; Nishimoto, S.; Ou, K.; Nishimura, T. Formation of Carbocycles via a 1,4-Rh Shift Triggered by a Rhodium-Catalyzed Addition of Terminal Alkynes to 3,3-Diarylcyclopropenes. Org. Lett. 2015, 17, 2630–2633; (d) Dou, X.; Huang, Y.; Hayashi, T. Asymmetric Conjugate Alkynylation of Cyclic α,β-Unsaturated Carbonyl Compounds with a Chiral Diene Rhodium Catalyst. Angew. Chem. Int. Ed. 2016, 55, 1133–1137; (e) Zhi, Y.; Huang, J.; Liu, N.; Lu, T.; Dou, X. Rhodium-Catalyzed Asymmetric Conjugate Alkynylation of β,γ-Unsaturated alpha-Ketoesters. Org. Lett. 2017, 19, 2378–2381.
- 7(a) Yazaki, R.; Kumagai, N.; Shibasaki, M. Direct Catalytic Asymmetric Conjugate Addition of Terminal Alkynes to α,β-Unsaturated Thioamides. J. Am. Chem. Soc. 2010, 132, 10275–10277; (b) Fan, B.-M.; Yang, Q.-J.; Hu, J.; Fan, C.-L.; Li, S.-F.; Yu, L.; Huang, C.; Tsang, W. W.; Kwong, F. Y. Asymmetric hydroalkynylation of norbornadienes promoted by chiral iridium catalysts. Angew. Chem. Int. Ed. 2012, 51, 7821–7823; (c) Bai, X.-Y.; Wang, Z.-X.; Li, B.-J. Iridium-Catalyzed Enantioselective Hydroalkynylation of Enamides for the Synthesis of Homopropargyl Amides. Angew. Chem. Int. Ed. 2016, 55, 9153–9157; (d) Wang, Z.-X.; Bai, X.-Y.; Yao, H.-C.; Li, B.-J. Synthesis of Amides with Remote Stereocenters by Catalytic Asymmetric γ-Alkynylation of α,β-Unsaturated Amides. J. Am. Chem. Soc. 2016, 138, 14872–14875; (e) Wang, Z.-X.; Li, B.-J. Construction of Acyclic Quaternary Carbon Stereocenters by Catalytic Asymmetric Hydroalkynylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 9312–9320; (f) Wang, Z.-X.; Li, B.-J. Iridium-Catalyzed Regiodivergent and Enantioselective Hydroalkynylation of Unactivated 1,1-Disubstituted Alkenes. Angew. Chem. Int. Ed. 2022, 61, e2022010.
- 8(a) Sanz-Marco, A.; Garcia-Ortiz, A.; Blay, G.; Pedro, J. R. Catalytic asymmetric conjugate addition of terminal alkynes to β-trifluoromethyl α,β-enones. Chem. Commun. 2014, 50, 2275–2278; (b) Sanz-Marco, A.; Blay, G.; Munoz, M. C.; Pedro, J. R. Highly enantioselective copper(I)-catalyzed conjugate addition of 1,3-diynes to alpha,beta-unsaturated trifluoromethyl ketones. Chem. Commun. 2015, 51, 8958–8961; (c) Mishra, S.; Liu, J.; Aponick, A. Enantioselective Alkyne Conjugate Addition Enabled by Readily Tuned Atropisomeric P, N-Ligands. J. Am. Chem. Soc. 2017, 139, 3352–3355.
- 9(a) Nishimura, T.; Sawano, T.; Ou, K.; Hayashi, T. Cobalt-catalyzed conjugate addition of silylacetylenes to α,β-unsaturated ketones. Chem. Commun. 2011, 47, 10142–10144; (b) Sawano, T.; Ashouri, A.; Nishimura, T.; Hayashi, T. Cobalt-catalyzed asymmetric 1,6-addition of (triisopropylsilyl)-acetylene to α,β,γ,δ-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2012, 134, 18936–18939; (c) Sawano, T.; Ou, K.; Nishimura, T.; Hayashi, T. Cobalt-catalyzed asymmetric addition of silylacetylenes to oxa- and azabenzonorbornadienes. Chem. Commun. 2012, 48, 6106–6108.
- 10(a) Hosseyni, S.; Smith, C. A.; Shi, X. Gold-Catalyzed Vinyl Ether Hydroalkynylation: An Alternative Pathway for the Gold-Catalyzed Intermolecular Reaction of Alkenes and Alkynes. Org. Lett. 2016, 18, 6336–6339; (b) Shen, Y.; Huang, B.; Zheng, J.; Lin, C.; Liu, Y.; Cui, S. Csp-Csp(3) Bond Formation via Iron(III)-Promoted Hydroalkynylation of Unactivated Alkenes. Org. Lett. 2017, 19, 1744–1747.
- 11 Jiang, X.; Han, B.; Xue, Y.; Duan, M.; Gui, Z.; Wang, Y.; Zhu, S. Nickel-Catalysed Migratory Hydroalkynylation and Enantioselective Hydroalkynylation of Olefins with Bromoalkynes. Nat. Commun. 2021, 12, 3792.
- 12 Yin, G.; Mu, X.; Liu, G. Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center. Acc. Chem. Res. 2016, 49, 2413–2423.
- 13(a) Qi, X.; Chen, C.; Hou, C.; Fu, L.; Chen, P.; Liu, G. Enantioselective Pd(II)-Catalyzed Intramolecular Oxidative 6-endo Aminoacetoxylation of Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 7415–7419; (b) Hou, C.; Chen, P.; Liu, G. Enantioselective Palladium(II)-Catalyzed Oxidative Aminofluorination of Unactivated Alkenes with Et4NF•3HF as a Fluoride Source. Angew. Chem. Int. Ed. 2020, 59, 2735–2739; (c) Chen, C.; Pflüger, P. M.; Chen, P.; Liu, G. Palladium(II)-Catalyzed Enantioselective Aminotrifluoromethoxylation of Unactivated Alkenes using CsOCF3 as a Trifluoromethoxide Source. Angew. Chem. Int. Ed. 2019, 58, 2392–2396; (d) Li, X.; Qi, X.; Hou, C.; Chen, P.; Liu, G. Palladium(II)-Catalyzed Enantioselective Azidation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2020, 59, 17239–17244; (e) Tian, B.; Chen, P.; Leng, X.; Liu, G. Palladium-catalysed enantioselective diacetoxylation of terminal alkenes. Nat. Catal. 2021, 4, 172–179; (f) Li, X.; Yang, T.; Li, J.; Li, X.; Chen, P.; Li, Z.; Liu, G. Regio- and enantioselective remote dioxygenation of internal alkenes. Nat. Chem. 2023, 15, 862–871.
- 14 Li, X.; Chen, P.; Liu, G. Palladium-catalyzed intermolecular alkynylcarbonylation of unactivated alkenes: easy access to beta-alkynylcarboxylic esters. Chem. Commun. 2022, 58, 2544–2547.
- 15 Yang, X.; Li, X.; Chen, P.; Liu, G. Palladium(II)-Catalyzed Enantioselective Hydrooxygenation of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2022, 144, 7972–7977.
- 16(a) Li, X.; Jin, J.; Chen, P.; Liu, G. Catalytic remote hydrohalogenation of internal alkenes. Nat. Chem. 2022, 14, 425–432; (b) Li, X.; Yang, X.; Chen, P.; Liu, G. Palladium-Catalyzed Remote Hydro-Oxygenation of Internal Alkenes: An Efficient Access to Primary Alcohols. J. Am. Chem. Soc. 2022, 144, 22877–22883.
- 17 Yoshimura, A.; Zhdankin, V. V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435.




