Impact of Polymer Matrix on Polymer Mechanochromism from Excited State Intramolecular Proton Transfer†
Yu Wu
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorXin Cheng
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorHuan Hu
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorShui Hu
Center of Advanced Elastomer Materials, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorZhimin Ma
College of Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Zhiyong Ma
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]Search for more papers by this authorYu Wu
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorXin Cheng
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorHuan Hu
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorShui Hu
Center of Advanced Elastomer Materials, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorZhimin Ma
College of Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Zhiyong Ma
Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Themes in Polymer Science.
Comprehensive Summary
Mechanochromic polymers based on non-covalent changes have attracted much attention recently. Herein, we report the impact of inter/intramolecular hydrogen bonds on polymer mechanochromism from the excited state intramolecular proton transfer (ESIPT) process. PhMz-NH2-OH and PhMz=2A are designed and obtained by simple and high-yield synthesis, and are connected into polyurethane and poly(methyl acrylate-co-2-ethylhexyl acrylate), respectively. In the initial state, the PhMz-NH2-OH@PU sample shows blue fluorescence from the excited enol form (E*) excitons, owing to intermolecular hydrogen bonds that interrupt the ESIPT reactions but the PhMz=2A@PMA-2-EA sample expresses cyan fluorescence belonging to the excited keto form (K*) emission, implying that the intramolecular hydrogen bonds matter. Furthermore, under stretching, external force can tune the emission of the PhMz=2A@PMA-2-EA sample from K* to E* state. Though external force can putatively still promote a bond rotation, ESIPT reactions remain equivalently interrupted in both the relaxed and stressed states in a hydrogen-bond donating environment. DFT calculation confirms the force-induced increase in dihedral angle for the transition of ESIPT-on/off. Thus, PhMz-NH2-OH@PU and PhMz=2A@PMA-2-EA showed disparate initial ESIPT states and further different responses/sensitivity to force. This study reports a novel and efficient strategy for enriching mechanochromic investigation and extending the applications of ESIPT reactions.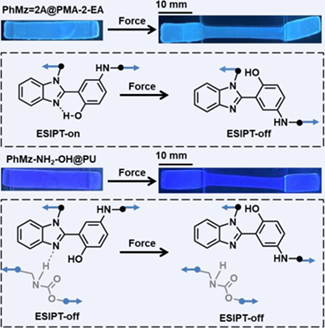
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300451-sup-0001-Supinfo.pdfPDF document, 2.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Chen, Y.; Mellot, G.; van Luijk, D.; Creton, C.Sijbesma, R. P. Mechanochemical tools for polymer materials. Chem. Soc. Rev. 2021, 50, 4100–4140.
- 2 Davis, D. A.; Hamilton, A.; Yang, J.; Cremar, L. D.; Van Gough, D.; Potisek, S. L.; Ong, M. T.; Braun, P. V.; Martinez, T. J.; White, S. R.; Moore, J. S.; Sottos, N. R. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72.
- 3 Lin, Y.; Barbee, M. H.; Chang, C. C.; Craig, S. L. Regiochemical Effects on Mechanophore Activation in Bulk Materials. J. Am. Chem. Soc. 2018, 140, 15969–15975.
- 4 Wang, Z.; Ma, Z.; Wang, Y.; Xu, Z.; Luo, Y.; Wei, Y.; Jia, X. A Novel Mechanochromic and Photochromic Polymer Film: When Rhodamine Joins Polyurethane. Adv. Mater. 2015, 27, 6469–6474.
- 5 Deng, Y.; Yuan, Y.; Chen, Y. Covalently Cross-Linked and Mechanochemiluminescent Polyolefins Capable of Self-Healing and Self-Reporting. CCS Chem. 2021, 3, 1316–1324.
- 6 Overholts, A. C.; Razo, W. G.; Robb, M. J. Mechanically gated formation of donor-acceptor Stenhouse adducts enabling mechanochemical multicolour soft lithography. Nat. Chem. 2023, 15, 332–338.
- 7 Qian, H.; Purwanto, N. S.; Ivanoff, D. G.; Halmes, A. J.; Sottos, N. R.; Moore, J. S. Fast, reversible mechanochromism of regioisomeric oxazine mechanophores: Developing in situ responsive force probes for polymeric materials. Chem 2021, 7, 1080–1091.
- 8 Hu, H.; Ma, Z.; Jia, X. Reaction Cascades in Polymer Mechanochemistry. Mater. Chem. Front. 2020, 4, 3115–3129.
- 9 Sagara, Y.; Karman, M.; Verde-Sesto, E.; Matsuo, K.; Kim, Y.; Tamaoki, N.; Weder, C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587.
- 10 Muramatsu, T.; Okado, Y.; Traeger, H.; Schrettl, S.; Tamaoki, N.; Weder, C.; Sagara, Y. Rotaxane-Based Dual Function Mechanophores Exhibiting Reversible and Irreversible Responses. J. Am. Chem. Soc. 2021, 143, 9884–9892.
- 11 Yang, J.; Xia, Y. Force-Triggered Guest Release from Mechanophore Incorporated Rotaxanes. CCS Chem. 2023, 5, 1365–1371.
- 12 Traeger, H.; Sagara, Y.; Kiebala, D. J.; Schrettl, S.; Weder, C. Folded Perylene Diimide Loops as Mechanoresponsive Motifs. Angew. Chem. Int. Ed. 2021, 60, 16191–16199.
- 13 Traeger, H.; Sagara, Y.; Berrocal, J. A.; Schrettl, S.; Weder, C. Strain-correlated mechanochromism in different polyurethanes featuring a supramolecular mechanophore. Polym. Chem. 2022, 13, 2860–2869.
- 14 Sagara, Y.; Traeger, H.; Li, J.; Okado, Y.; Schrettl, S.; Tamaoki, N.; Weder, C. Mechanically Responsive Luminescent Polymers Based on Supramolecular Cyclophane Mechanophores. J. Am. Chem. Soc. 2021, 143, 5519–5525.
- 15 Kotani, R.; Yokoyama, S.; Nobusue, S.; Yamaguchi, S.; Osuka, A.; Yabu, H.; Saito, S. Bridging pico-to-nanonewtons with a ratiometric force probe for monitoring nanoscale polymer physics before damage. Nat. Commun. 2022, 13, 303.
- 16 Kimura, R.; Kitakado, H.; Yamakado, T.; Yoshida, H.; Saito, S. Probing a microviscosity change at the nematic-isotropic liquid crystal phase transition by a ratiometric flapping fluorophore. Chem. Commun. 2022, 58, 2128–2131.
- 17 Yamakado, T.; Saito, S. Ratiometric Flapping Force Probe That Works in Polymer Gels. J. Am. Chem. Soc. 2022, 144, 2804–2815.
- 18 Raisch, M.; Maftuhin, W.; Walter, M.; Sommer, M. A mechanochromic donor-acceptor torsional spring. Nat. Commun. 2021, 12, 4243.
- 19 Raisch, M.; Reiter, G.; Sommer, M. Determining Entanglement Molar Mass of Glassy Polyphenylenes Using Mechanochromic Molecular Springs. ACS Macro Lett. 2022, 11, 760–765.
- 20 Hertel, R.; Maftuhin, W.; Walter, M.; Sommer, M. Conformer Ring Flip Enhances Mechanochromic Performance of ansa-Donor-Acceptor-Donor Mechanochromic Torsional Springs. J. Am. Chem. Soc. 2022, 144, 21897–21907.
- 21 Hu, H.; Cheng, X.; Ma, Z.; Sijbesma, R. P.; Ma, Z. Polymer Mechanochromism from Force-Tuned Excited-State Intramolecular Proton Transfer. J. Am. Chem. Soc. 2022, 144, 9971–9979.
- 22 Cheng, X.; Hu, H.; Wu, Y.; Ma, Z.; Ma, Z. Photo-gated polymer mechanochromism from excited-state intramolecular proton transfer. Chem. Commun. 2023, 59, 7236–7239.
- 23 Huang, Q.; Guo, Q.; Lan, J.; Su, R.; Ran, Y.; Yang, Y.; Bin, Z.; You, J. Mechanically induced single-molecule white-light emission of excited-state intramolecular proton transfer (ESIPT) materials. Mater. Horiz. 2021, 8, 1499–1508.
- 24 Karman, M.; Verde-Sesto, E.; Weder, C. Mechanochemical Activation of Polymer-Embedded Photoluminescent Benzoxazole Moieties. ACS Macro Lett. 2018, 7, 1028–1033.
- 25 Padalkar, V. S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202.
- 26 Demchenko, A. P.; Tang, K. C.; Chou, P. T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408.
- 27 Sedgwick, A. C.; Wu, L.; Han, H. H.; Bull, S. D.; He, X. P.; James, T. D.; Sessler, J. L.; Tang, B. Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880.
- 28 Kwon, J. E.; Park, S. Y. Advanced organic optoelectronic materials: harnessing excited-state intramolecular proton transfer (ESIPT) process. Adv. Mater. 2011, 23, 3615–3642.
- 29 Chen, C.-L.; Chen, Y.-T.; Demchenko, A. P.; Chou, P.-T. Amino proton donors in excited-state intramolecular proton-transfer reactions. Nat. Rev. Chem. 2018, 2, 131–143.
- 30 Yi, S. Z.; Li, B. N.; Fu, P. Y.; Pan, M.; Su, C. Y. Interplay of Dual-Proton Transfer Relay to Achieve Full-Color Panel Luminescence in Excited-State Intramolecular Proton Transfer (ESIPT) Fluorophores. ACS Appl. Mater. Interfaces 2023, 15, 3172–3181.
- 31 Szemik-Hojniak, A.; Wisniewski, L.; Deperasinska, I.; Makarewicz, A.; Jerzykiewicz, L.; Puszko, A.; Erez, Y.; Huppert, D. The impact of solvent polarity on intramolecular proton and electron transfer in 2-alkylamino-4-nitro-5-methyl pyridine N-oxides. Phys. Chem. Chem. Phys. 2012, 14, 8147–8159.
- 32 Gu, H.; Wang, W.; Wu, W.; Wang, M.; Liu, Y.; Jiao, Y.; Wang, F.; Wang, F.; Chen, X. Excited-state intramolecular proton transfer (ESIPT)- based fluorescent probes for biomarker detection: design, mechanism, and application. Chem. Commun. 2023, 59, 2056–2071.
- 33 Wang, K.; Feng, B.; Wang, G.; Cui, J.; Yang, L.; Jiang, K.; Zhang, H. A specific esterase and pH logically regulate ESIPT: different kinds of granulocyte sorting. Chem. Commun. 2022, 58, 2894–2897.
- 34 Kim, S.; Seo, J.; Jung, H. K.; Kim, J. J.; Park, S. Y. White Luminescence from Polymer Thin Films Containing Excited-State Intramolecular Proton-Transfer Dyes. Adv. Mater. 2005, 17, 2077–2082.
- 35 Furukawa, S.; Shono, H.; Mutai, T.; Araki, K. Colorless, transparent, dye-doped polymer films exhibiting tunable luminescence color: controlling the dual-color luminescence of 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine derivatives with the surrounding matrix. ACS Appl. Mater. Interfaces 2014, 6, 16065–16070.
- 36 Yang, C.-C.; Tian, Y.; Chen, C.-Y.; Jen, A. K. Y.; Chen, W.-C. A Novel Benzoxazole-Containing Poly(N-isopropylacrylamide) Copolymer as a Multifunctional Sensing Material. Macromol. Rapid Commun. 2007, 28, 894–899.
- 37 Campo, L. F.; Corrêa, D. S.; Araújo, M. A. d.; Stefani, V. New fluorescent monomers and polymers displaying an intramolecular proton-transfer mechanism in the electronically excited state (ESIPT), 1. Synthesis of benzazolylvinylene derivatives and its copolymerization with methyl methacrylate (MMA). Macromol. Rapid Commun. 2000, 21, 832–836.
- 38 Huang, Y.; Lv, T.; Qin, T.; Xu, Z.; Wang, L.; Liu, B. A DS2-specific flavonoid-based probe with a unique dual-emissive response to human serum albumin. Chem. Commun. 2020, 56, 11094–11097.
- 39 Nakane, Y.; Takeda, T.; Hoshino, N.; Sakai, K.-i.Akutagawa, T. ESIPT Fluorescent Chromism and Conformational Change of 3-(2-Benzothiazolyl)-4-hydroxy-benzenesulfonic acid by Amine Sorption. J. Phys. Chem. C 2018, 122, 16249–16255.
- 40 Cheng, X.; Hu, H.; Wu, Y.; Ma, Z.; Ma, Z. Photoinduced Clusteroluminescence Redshift of Poly(methyl acrylate) via Radicals. ACS Appl. Mater. Interfaces 2022, 14, 56185–56192.
- 41 Klein, I. M.; Husic, C. C.; Kovacs, D. P.; Choquette, N. J.; Robb, M. J. Validation of the CoGEF Method as a Predictive Tool for Polymer Mechanochemistry. J. Am. Chem. Soc. 2020, 142, 16364–16381.




