Nickel/Quinim Enabled Asymmetric Carbamoyl-Acylation of Unactivated Alkenes
Xianqing Wu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally.
Search for more papers by this authorHaiyan Li
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally.
Search for more papers by this authorFeng He
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorJingping Qu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yifeng Chen
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorXianqing Wu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally.
Search for more papers by this authorHaiyan Li
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally.
Search for more papers by this authorFeng He
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorJingping Qu
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yifeng Chen
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
Transition metal-catalyzed difunctionalization of tethered alkene has emerged as a prevailing tool for the expedient construction of synthetically valuable cyclic compounds. However, most efforts have been devoted to the reaction of styrene-type substrates due to their rigid scaffold and high reactivity. With respect to the difunctionalization of nonaromatic tethered olefin, especially the mono-substituted alkene, still remains largely underdeveloped. Herein, we disclose a nickel/Quinim complex and TBADT-cocatalyzed asymmetric carbamoyl-acylation of unactivated alkene tethered on nonaromatic carbamoyl chlorides with diverse aldehydes. The reaction exhibits broad substrate scope with good functional group tolerance, as well as high reaction efficiency and enantioselectivity. Both monosubstituted and 1,1-substituted alkenes can work well with either aliphatic or aromatic aldehydes under the current protocol, providing convenient access to an array of medicinally useful chiral γ-lactams derivatives bearing a convertible acyl functionality. This reaction showcases more application possibilities of the chiral Quinim ligand in the future asymmetric catalytic transformations.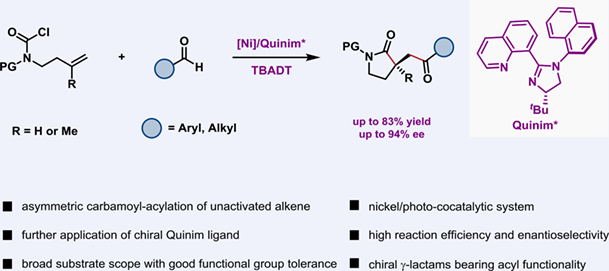
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200856-sup-0001-supinfo.pdfPDF document, 4.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274; (b) Ye, L.-W.; Shu, C.; Gagosz, F. Recent Progress Towards Transition Metal-Catalyzed Aynthesis of γ-Lactams. Org. Biomol. Chem. 2014, 12, 1833–1845.
- 2For selected reviews, see: (a) Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340; (b) Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of Quaternary Stereocenters by Palladium Catalyzed Carbopalladation-Initiated Cascade Reactions. Angew. Chem. Int. Ed. 2019, 58, 1562–1573; (c) Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371–1394; (d) Qi, X.; Diao, T. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. ACS Catal. 2020, 10, 8542–8556; (e) Poremba, K. E.; Dibrell, S. E.; Reisman, S. E. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10, 8237–8246; (f) Ping, Y.; Song, H.; Kong, W. Recent Advances in Ni-Catalyzed Asymmetric Reductive Difunctionalization of Alkenes. Chin. J. Org. Chem. 2022, 42, 3302–3321.
- 3For selected examples on transition metal-catalyzed enantioselective redox-neutral dicarbofunctionalization of tethered alkene, see: (a) Watson, M. P.; Jacobsen, E. N. Asymmetric Intramolecular Arylcyanation of Unactivated Olefins via C-CN Bond Activation. J. Am. Chem. Soc. 2008, 130, 12594–12595; (b) Nakao, Y.; Ebata, S.; Yada, A.; Hiyama, T.; Ikawa, M.; Ogoshi, S. Intramolecular Arylcyanation of Alkenes Catalyzed by Nickel/AlMe2Cl. J. Am. Chem. Soc. 2008, 130, 12874–12875; (c) Cong, H.; Fu, G. C. Catalytic Enantioselective Cyclization/Cross-Coupling with Alkyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 3788–3791; (d) You, W.; Brown, M. K. Catalytic Enantioselective Diarylation of Alkenes. J. Am. Chem. Soc. 2015, 137, 14578–14581; (e) Chierchia, M.; Xu, P.; Lovinger, G. J. Morken, J. P. Enantioselective Radical Addition/Cross-Coupling of Organozinc Reagents, Alkyl Iodide, and Alkynyl Boron Reagents. Angew. Chem. Int. Ed. 2019, 58, 14245–14249; (f) Yasui, Y.; Kamisaki, H.; Takemoto, Y. Enantioselective Synthesis of 3,3-Disubstituted Oxindoles Through Pd-Catalyzed Cyanoamidation. Org. Lett. 2008, 10, 3303–3306; (g) Ju, B.; Chen, S.; Kong, W. Enantioselective Palladium-Catalyzed Diarylation of Unactivated Alkenes. Chem. Commun. 2019, 55, 14311–14314; (h) Zhang, Z.-M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y.; Zhang, J. Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction. Angew. Chem. Int. Ed. 2019, 58, 14653–14659.
- 4For selected examples on transition metal-catalyzed enantioselective reductive dicarbofunctionalization of tethered alkene, see: (a) Wang, K.; Ding, Z.; Zhou, Z.; Kong, W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross- Coupling. J. Am. Chem. Soc. 2018, 140, 12364–12368; (b) Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Reductive Arylalkylation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2019, 58, 6722–6726; (c) Tian, Z.-X.; Qiao, J.-B.; Xu, G.-L.; Pang, X.; Qi, L.; Ma, W.-Y.; Zhao, Z.-Z.; Duan, J.; Du, Y.-F.; Su, P.; Liu, X.-Y.; Shu, X.-Z. Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 7637–7643; (d) Peng, Y.; Wang, K.; Pan, Q.; Ding, Z.; Zhou, Z.; Guo, Y.; Kong, W. Ni-Catalyzed Regio- and Enantioselective Domino Reductive Cyclization: One-Pot Synthesis of 2,3-Fused CyclopentannulatedIndolines. ACS Catal. 2019, 9, 7335–7342. (e) Li, Y.; Ding, Z.; Lei, A.; Kong, W. Ni-Catalyzed Enantioselective Reductive Aryl-Alkenylation of Alkenes: Application to the Synthesis of (+)-Physovenine and (+)-Physostigmine. Org. Chem. Front. 2019, 6, 3305–3309. (f) He, J.; Xue, Y.; Han, B.; Zhang, C.; Wang, Y.; Zhu, S. Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem. Int. Ed. 2020, 59, 2328–2332; (g) Wu, J.; Wang, C. Nickel-Catalyzed Asymmetric Reductive Dicarbamoylation of Alkenes. Org. Lett. 2021, 23, 6407–6411; (h) Zhao, T.-Y.; Xiao, L.-J.; Zhou, Q.-L. Nickel-Catalyzed Desymmetric Reductive Cyclization/Coupling of 1,6-Dienes: An Enantioselective Approach to Chiral Tertiary Alcohol. Angew. Chem. Int. Ed. 2022, 61, e202115702.
- 5 Li, Y.; Zhang, F.-P.; Wang, R.-H.; Qi, S.-L.; Luan, Y.-X.; Ye, M. Carbamoyl Fluoride-Enabled Enantioselective Ni-Catalyzed Carbocarbamoylation of Unactivated Alkenes. J. Am. Chem. Soc. 2020, 142, 19844–19849.
- 6(a) Wu, X.; Qu, J.; Chen, Y. Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam. J. Am. Chem. Soc. 2020, 142, 15654–15660; (b) Wu, X.; Luan, B.; Zhao, W.; He, F.; Wu, X.-Y.; Qu, J.; Chen, Y. Catalytic Desymmetric Dicarbofunctionalization of Unactivated Alkenes. Angew. Chem. Int. Ed. 2022, 61, e2021115983; (c) Wu, X.; Turlik, A.; Luan, B.; He, F.; Qu, J.; Houk, K. N.; Chen, Y. Nickel-Catalyzed Enantioselective Reductive Alkyl-Carbamoylation of Internal Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202207536; (d) He, F.; Hou, L.; Wu, X.; Ding, H.; Qu, J.; Chen, Y. Enantioselective Synthesis of α-Alkenylated γ-Lactam Enabled by Ni-Catalyzed 1,4-Arycarbamoylation of 1,3-Dienes. CCS Chem. 2023, 5, 341–349; (e) Zhang C.; Wu, X.; Xia, T.; Qu, J.; Chen, Y. Ni-catalyzed Carbamoylation of Unactivated Alkenes for Stereoselective Construction of Six-Membered Lactams. Nat. Commun. 2022, 13, 5964–5972.
- 7 Torelli, A.; Whyte, A.; Polishchuk, I.; Bajohr, J.; Lautens, M. Stereoselective Construction of γ-Lactams via Copper-Catalyzed Borylacylation. Org. Lett. 2020, 22, 7915–7919.
- 8 Yasui, Y.; Kamisaki, H.; Ishida, T.; Takemoto, Y. Synthesis of 3,3-Disubstituted Oxindoles through Pd-Catalyzed Intramolecular Cyanoamidation. Tetrahedron 2010, 66, 1980–1989.
- 9 Feng, Z.; Li, Q.; Chen, L.; Yao, H.; Lin, A. Palladium-Catalyzed Asymmetric Carbamoyl-Carbonylation of Alkenes. Sci. China Chem. 2021, 64, 1367–1371.
- 10(a) Qiao, J.-B.; Zhang, Y.-Q.; Yao, Q.-W.; Zhao, Z.-Z.; Peng, X.; Shu, X.-Z. Enantioselective Reductive Divinylation of Unactivated Alkenes by Nickel-Catalyzed Cyclization Coupling Reaction. J. Am. Chem. Soc. 2021, 143, 12961–12967; (b) Jia, X.-G.; Yao, Q.-W.; Shu, X.-Z. Enantioselective Reductive N-Cyclization–Alkylation Reaction of Alkene- Tethered Oxime Esters and Alkyl Iodides by Nickel Catalysis. J. Am. Chem. Soc. 2022, 144, 13461–13467.
- 11For selected reviews, see: (a) Tzirakis, M. D.; Lykakis, I. N.; Orfanopoulos, M. Decatungstate as an Efficient Photocatalyst in Organic Chemistry. Chem. Soc. Rev. 2009, 38, 2609–2621; (b) Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-Selective C–H Functionalization by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 2018, 8, 701–713; (c) Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C−H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924.
- 12(a) Esposti, S.; Dondi, D.; Fagnoni, M.; Albini, A. Acylation of Electrophilic Olefins through Decatungstate-Photocatalyzed Activation of Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 2531–2534; (b) Fan, P.; Zhang, C.; Lan, Y.; Lin, Z.; Zhang, L.; Wang, C. Photocatalytic Hydroacylation of Trifluoromethyl Alkenes. Chem. Commun. 2019, 55, 12691–12694; (c) Wang, X.; Chen, Y.; Song, H.; Liu, Y.; Wang, Q. Synthesis of Unnatural α-Amino Acids via Photoinduced Decatungstate- Catalyzed Giese Reactions of Aldehydes. Org. Lett. 2021, 23, 2199–2204; (e) Le, S.; Li, J.; Feng, J.; Zhang, Z.; Bai, Y.; Yuan, Z.; Zhu, G. [3+2] Cycloaddition of Alkyl Aldehydes and Alkynes Enabled by Photoinduced Hydrogen Atom Transfer. Nat. Commun. 2022, 13, 4734–4741.
- 13(a) Meanwell, M.; Lehmann, J.; Eichenberger, M.; Martin, R. E.; Britton, R. Synthesis of Acyl Fluorides via Photocatalytic Fluorination of Aldehydic C–H Bonds. Chem. Commun. 2018, 54, 9985–9988; (b) Dong, J.; Wang, X.; Wang, Z.; Song, H.; Liu, Y.; Wang, Q. Formyl-Selective Deuteration of Aldehydes with D2O via Synergistic Organic and Photoredox Catalysis. Chem. Sci. 2020, 11, 1026–1031; (c) Kuang, Y.; Cao, H.; Tang, H.; Chew, J.; Chen, W.; Shi, X.; Wu, J. Visible Light Driven Deuteration of Formyl C–H and Hydridic C(sp3)–H Bonds in Feedstock Chemicals and Pharmaceutical Molecules. Chem. Sci. 2020, 11, 8912–8918.
- 14 Perry, I. B.; Brewer, T. F.; Sarver, P. J.; Schultz, D. M.; DiRocco, D. A.; MacMillan, D. W. C. Direct Arylation of Strong Aliphatic C–H Bonds. Nature 2018, 560, 70–75.
- 15 Fan, P.; Lan, Y.; Zhang, C.; Wang, C. Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes. J. Am. Chem. Soc. 2020, 142, 2180–2186.
- 16(a) Fan, P.; Zhang, C.; Zhang, L.; Wang, C. Acylation of Aryl Halides and α-Bromo Acetates with Aldehydes Enabled by Nickel/TBADT Cocatalysis. Org. Lett. 2020, 22, 3875–3878; (b) Fan, P.; Jin, Y.; Liu, J.; Wang, R.; Wang, C. Nickel/Photo-Cocatalyzed Regioselective Ring Opening of N-Tosyl Styrenyl Aziridines with Aldehydes. Org. Lett. 2021, 23, 7364–7369; (c) Fan, P.; Wang, R.; Wang, C. Nickel/Photo- Cocatalyzed C(sp2)–H Allylation of Aldehydes and Formamides. Org. Lett. 2021, 23, 7672–7677; (d) Wang, R.; Fan, P.; Wang, C. Nickel/ Photo-Cocatalyzed Asymmetric Acyl C−H Allylation of Aldehydes and Formamides ACS Catal. 2023, 13, 141–146; (e) Murugesan, V.; Muralidharan, A.; Anantharaj, G. V.; Chinnusamy, T.; Rasappan, R. Photoredox−Ni Dual Catalysis: Chelation-Free Hydroacylation of Terminal Alkynes. Org. Lett. 2022, 24, 8435–8440; (f) Liu, W.; Ke, Y.; Liu, C.; Kong, W. Direct Acylation and Alkynylation of Hydrocarbons via Synergistic Decatungstate Photo-HAT/Nickel Catalysis. Chem. Commun. 2022, 58, 11937–11940; (g) Xu, S.; Chen, H.; Zhou, Z.; Kong, W. Three-Component Alkene Difunctionalization via Direct and Selective Activation of Aliphatic C-H Bonds. Angew. Chem. Int. Ed. 2021, 60, 7405–7411; (h) Wang, L.; Wang, T.; Cheng, G.-J.; Li, X.; Wei, J.-J.; Guo, B.; Zheng, C.; Chen, G.; Ran, C.; Zheng, C. Direct C–H Arylation of Aldehydes by Merging Photocatalyzed Hydrogen Atom Transfer with Palladium Catalysis. ACS Catal. 2020, 10, 7543–7551; (i) Fan, P.; Mao, Y.; Wang, C. Synthesis of 1,4-diketones via Palladium/Photo-Cocatalyzed Dehydrogenative Cross-Coupling. Org. Chem. Front. 2022, 9, 4649–4653.
- 17(a) Shrestha, M.; Wu, X.; Huang, W.; Qu, J.; Chen, Y. Recent Advances in Transition Metal-Catalyzed Reactions of Carbamoyl Chlorides. Org. Chem. Front. 2021, 8, 4024–4045; (b) Wang, C.; Zhao, W.; Wu, X.; Qu, J.; Chen, Y. Palladium-Catalyzed Regioselective Domino Spirocyclization of Carbamoyl Chlorides with Alkynes and Benzynes. Adv. Synth. Catal. 2020, 362, 4996–5001; (c) Zhang, C.; Wu, X.; Wang, C.; Zhang, C.; Qu, J.; Chen, Y. Pd/Cu-Catalyzed Domino Cyclization/Deborylation of Alkene-Tethered Carbamoyl Chloride and 1,1-Diborylmethane. Org. Lett. 2020, 22, 6376–6381; (d) Wu, X.; Tang, Z.; Zhang, C.; Wang, C.; Wu, L.; Qu, J.; Chen, Y. Pd-Catalyzed Regiodivergent Synthesis of Diverse Oxindoles Enabled by the Versatile Heck Reaction of Carbamoyl Chlorides. Org. Lett. 2020, 22, 3915–3921; (e) Luan, B.; Tang, Z.; Wu, X.; Chen, Y. 8-Quinolinyl Oxaoline: Ligand Exploration in Enantioselective Ni-Catalyzed Reductive Carbamoyl-Alkylation of Alkene to Access the Chiral Oxindoles. Synlett 2022, 33, 1847–1852.
- 18(a) Li, J.; Yu, B.; Lu, Z. Chiral Imidazoline Ligands and Their Applications in Metal-Catalyzed Asymmetric Synthesis. Chin. J. Chem. 2021, 39, 488–514; (b) Cheng, X.; Lu, H.; Lu, Z. Enantioselective Benzylic C–H Arylation via Photoredox and Nickel Dual Catalysis. Nat. Commun. 2019, 10, 3549–3555; (c) Li, X.; Cheng, X.; Lu, J.; Wang, H.; Fan, Q.; Lu, Z. Enantioselective Reductive Cross-Coupling of Aryl/Alkenyl Bromides with Benzylic Chlorides via Photoredox/Biimidazoline Nickel Dual Catalysis. Chin. J. Chem. 2022, 40, 1033–1038; (d) Wang, J.-Y.; Li, C.-L.; Xu, T.; Li, M.-F.; Hao, W.-J.; Tu, S.-J.; Wang, J.; Li, G.; Yu, Z.-X.; Jiang, B. Catalytic Enantioselective Construction of 6-4 Ring-Junction All-Carbon Stereocenters and Mechanistic Insights. Chin. J. Chem. 2022, 40, 1767–1776.




