Visible Light-Induced C-5 Selective C—H Radical Borylation of Imidazo[1,2-a]pyridines with NHC-Boranes
Huitao Zheng
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorHao Lu
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorChaobo Su
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorRunlong Yang
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorLimin Zhao
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Xiang Liu
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected], [email protected]Search for more papers by this authorHuitao Zheng
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorHao Lu
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorChaobo Su
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorRunlong Yang
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorLimin Zhao
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Xiang Liu
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering and Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
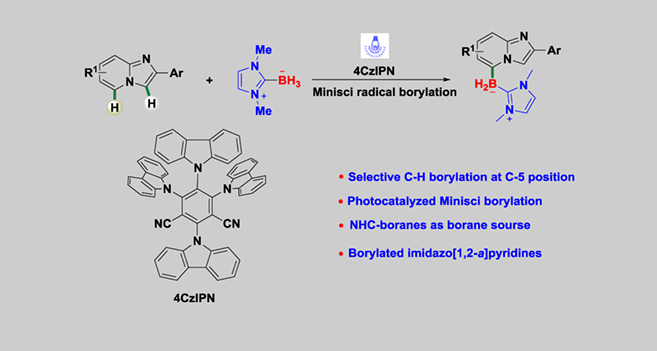
A visible-light-induced C-5 selective C—H borylation of imidazo[1,2-a]pyridines with NHC-BH3 via Minisci-type radical borylation reaction has been developed for the first time. The present sustainable protocol provides a new family of regioselectively C5-borylated imidazopyridines that would otherwise be difficult to prepare. It is a supplement to site-selective borylation of azines (nitrogen-containing aromatic heterocycles) and the assembly of sp2 carbon-boron bond.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200568-sup-0001-Supinfo.pdfPDF document, 4.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Starrett, J.; Montzka, T.; Crosswell, A.; Cavanagh, R. Synthesis and biological activity of 3-substituted imidazo [1,2-a] pyridines as antiulcer agents. J. Med. Chem. 1989, 32, 2204–2210; (b) Quattrini, L.; Gelardi, E. L. M.; Coviello, V.; Sartini, S. D.; Ferraris, M.; Mori, M.; Nakano, I.; Garavaglia, S.; La Motta, C. Imidazo [1,2-a] pyridine derivatives as aldehyde dehydrogenase inhibitors: Novel chemotypes to target glioblastoma stem cells. J. Med. Chem. 2020, 63, 4603–4616.
- 2(a) Al-Tel, T. H.; Al-Qawasmeh, R. A.; Zaarour, R. Design, synthesis and in vitro antimicrobial evaluation of novel Imidazo[1,2-a]pyridine and imidazo[2,1-b][1,3]benzothiazole motifs. Eur. J. Med. Chem. 2011, 46, 1874–1881; (b) Yu, Y.; Han, Y.; Zhang, F.; Gao, Z.; Zhu, T.; Dong, S.; Ma, M. Design, Synthesis, and Biological Evaluation of Imidazo[1,2-a]pyridine Derivatives as Novel PI3K/mTOR Dual Inhibitors. J. Med. Chem. 2020, 63, 3028–3046.
- 3(a) Gudmundsson, K. S.; Johns, B. A. Imidazo[1,2-a]pyridines with potent activity against herpesviruses. Bioorg. Med. Chem. Lett. 2007, 17, 2735–2739; (b) Gudmundsson, K. S.; Williams, J. D.; Drach, J. C.; Townsend, L. B. Synthesis and antiviral activity of novel erythrofuranosyl imidazo [1,2-a] pyridine C-nucleosides constructed via palladium coupling of iodoimidazo [1,2-a] pyridines and dihydrofuran. J. Med. Chem. 2003, 46, 1449–1455.
- 4 Enguehard-Gueiffier, C.; Fauvelle, F.; Debouzy, J. C.; Peinnequin, A.; Thery, I.; Dabouis, V.; Gueiffier, A. 2,3-Diarylimidazo[1,2-a]pyridines as potential inhibitors of UV-induced keratinocytes apoptosis: synthesis, pharmacological properties and interactions with model membranes and oligonucleotides by NMR. Eur. J. Pharm. Sci. 2005, 24, 219–227.
- 5(a) Enguehard-Gueiffier, C.; Gueiffier, A. Recent progress in the pharmacology of imidazo [1,2-a] pyridines. Mini-Rev. Med. Chem. 2007, 7, 888–899; (b) Bagdi, A. K.; Santra, S.; Monir, K.; Hajra, A. Synthesis of imidazo[1,2-a]pyridines: a decade update. Chem. Commun. 2015, 51, 1555–1575; (c) Bagdi, A. K.; Hajra, A. Design, synthesis, and functionalization of imidazoheterocycles. Chem. Rec. 2016, 16, 1868–1885.
- 6(a) Liu, P.; Gao, Y.; Gu, W.; Shen, Z.; Sun, P. Regioselective Fluorination of Imidazo[1,2-a]pyridines with Selectfluor in Aqueous Condition. J. Org. Chem. 2015, 80, 11559–11565; (b) Yan, K.; Yang, D.; Wei, W.; Lu, S.; Li, G.; Zhao, C.; Zhang, Q.; Wang, H. Copper-catalyzed domino synthesis of benzo[b]thiophene/imidazo[1,2-a]pyridines by sequential Ullmann-type coupling and intramolecular C(sp2)–H thiolation. Org. Chem. Front. 2016, 3, 66–70; (c) Zhang, J. R.; Liao, Y. Y.; Deng, J. C.; Feng, K. Y.; Zhang, M.; Ning, Y. Y.; Lin, Z. W.; Tang, R. Y. Oxidative dual C-H thiolation of imidazopyridines with ethers or alkanes using elemental sulphur. Chem. Commun. 2017, 53, 7784–7787; (d) Yang, Q.; Li, S.; Wang, J. Cobalt-catalyzed cross-dehydrogenative coupling of imidazo[1,2-a]pyridines with isochroman using molecular oxygen as the oxidant. Org. Chem. Front. 2018, 5, 577–581; (e) Ren, Y.; Xu, B.; Zhong, Z.; Pittman, C. U.; Zhou, A. Using SeO2 as a selenium source to make RSe-substituted aniline and imidazo[1,2-a]pyridine derivatives. Org. Chem. Front. 2019, 6, 2023–2027; (f) Wu, Y.; Li, L.; Wen, K.; Deng, J.; Chen, J.; Shi, J.; Wu, T.; Pang, J.; Tang, X. Copper-Catalyzed C-3 Functionalization of Imidazo[1,2-a]pyridines with 3-Indoleacetic Acids. J. Org. Chem. 2021, 86, 12394–12402; (g) Chen, J.; Wen, K.; Wu, Y.; Shi, J.; Yao, X.; Tang, X. Transition Metal Catalyst-Free C-3 Sulfonylmethylation of Imidazo[1,2-a]pyridines with Glyoxylic Acid and Sodium Sulfinates in Water. J. Org. Chem. 2022, 87, 3780–3787.
- 7 Jin, S.; Xie, B.; Lin, S.; Min, C.; Deng, R.; Yan, Z. Metal-Free Site-Specific Hydroxyalkylation of Imidazo[1,2-a]pyridines with Alcohols through Radical Reaction. Org. Lett. 2019, 21, 3436–3440.
- 8(a) Jin, C.; Sun, B.; Xu, T.; Zhang, L.; Zhu, R.; Yang, J.; Xu, M. Metal- Free Regioselective Alkylation of Imidazo[1,2-a]pyridines with N-Hydroxyphthalimide Esters under Organic Photoredox Catalysis. Synlett 2020, 31, 363–368; (b) Samanta, S.; Hajra, A. Mn(II)-Catalyzed C-H Alkylation of Imidazopyridines and N-Heteroarenes via Decarbonylative and Cross-Dehydrogenative Coupling. J. Org. Chem. 2019, 84, 4363–4371; (c) Jin, S.; Yao, H.; Lin, S.; You, X.; Yang, Y.; Yan, Z. Peroxide-mediated site-specific C-H methylation of imidazo[1,2-a]pyridines and quinoxalin-2(1H)-ones under metal-free conditions. Org. Biomol. Chem. 2020, 18, 205–210.
- 9 Li, Y.; Shu, K.; Liu, P.; Sun, P. Selective C-5 Oxidative Radical Silylation of Imidazopyridines Promoted by Lewis Acid. Org. Lett. 2020, 22, 6304–6307.
- 10(a) Roughley, S. D.; Jordan, A. M. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479; (b) Fyfe, J. W.; Watson, A. J. Recent developments in organoboron chemistry: old dogs, new tricks. Chem 2017, 3, 31–55; (c) Neeve, E. C.; Geier, S. J.; Mkhalid, I. A.; Westcott, S. A.; Marder, T. B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161; (d) Cuenca, A. B.; Shishido, R.; Ito, H.; Fernandez, E. Transition-metal- free B-B and B-interelement reactions with organic molecules. Chem. Soc. Rev. 2017, 46, 415–430.
- 11(a) Mazzacano, T. J.; Mankad, N. P. Base metal catalysts for photochemical C–H borylation that utilize metal–metal cooperativity. J. Am. Chem. Soc. 2013, 135, 17258–17261; (b) Parmelee, S. R.; Mazzacano, T. J.; Zhu, Y.; Mankad, N. P.; Keith, J. A heterobimetallic mechanism for C–H borylation elucidated from experimental and computational data. ACS. Catal. 2015, 5, 3689–3699.
- 12(a) Shimada, S.; Batsanov, A. S.; Howard, J. A.; Marder, T. Formation of Aryl-and Benzylboronate Esters by Rhodium-Catalyzed C−H Bond Functionalization with Pinacolborane. Angew. Chem. Int. Ed. 2001, 40, 2168–2171;
10.1002/1521-3773(20010601)40:11<2168::AID-ANIE2168>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar(b) Tian, Y.-M.; Guo, X.-N.; Braunschweig, H.; Radius, U.; Marder, T. Photoinduced Borylation for the Synthesis of Organoboron Compounds: Focus Review. Chem. Rev. 2021, 121, 3561–3597.
- 13 Sadler, S. A.; Tajuddin, H.; Mkhalid, I. A.; Batsanov, A. S.; Albesa-Jove, D.; Cheung, M. S.; Maxwell, A. C.; Shukla, L.; Roberts, B.; Blakemore, D. C.; Lin, Z.; Marder, T. B.; Steel, P. G. Iridium-catalyzed C-H borylation of pyridines. Org. Biomol. Chem. 2014, 12, 7318–7327.
- 14(a) Yang, L.; Semba, K.; Nakao, Y. para-Selective C-H Borylation of (Hetero)Arenes by Cooperative Iridium/Aluminum Catalysis. Angew. Chem. Int. Ed. 2017, 56, 4853–4935; (b) Yang, L.; Uemura, N.; Nakao, Y. meta-Selective C-H Borylation of Benzamides and Pyridines by an Iridium-Lewis Acid Bifunctional Catalyst. J. Am. Chem. Soc. 2019, 141, 7972–7979; (c) Trouve, J.; Zardi, P.; Al-Shehimy, S.; Roisnel, T.; Gramage-Doria, R. Enzyme-like Supramolecular Iridium Catalysis Enabling C-H Bond Borylation of Pyridines with meta-Selectivity. Angew. Chem. Int. Ed. 2021, 60, 18006–18013.
- 15 Larsen, M. A.; Hartwig, J. F. Iridium-catalyzed C-H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism. J. Am. Chem. Soc. 2014, 136, 4287–4299.
- 16 Luo, Y.; Jiang, S.; Xu, X. Yttrium-Catalyzed ortho-Selective C-H Borylation of Pyridines with Pinacolborane. Angew. Chem. Int. Ed. 2022, e202117750.
- 17 Kim, J. H.; Constantin, T.; Simonetti, M.; Llaveria, J.; Sheikh, N. S.; Leonori, D. A radical approach for the selective C-H borylation of azines. Nature 2021, 595, 677–683.
- 18(a) Takahashi, K.; Geib, S. J.; Maeda, K.; Curran, D. P.; Taniguchi, T. Radical trans-hydroboration of substituted 1, 3-diynes with an N-heterocyclic carbene borane. Org. Lett. 2021, 23, 1071–1075;
(b) Jin, J.-K.; Zhang, F.-L.; Zhao, Q.; Lu, J.-A.; Wang, Y.-F. Synthesis of diverse boron-handled N-heterocycles via radical borylative cyclization of N-allylcyanamides. Org. Lett. 2018, 20, 7558–7562;
(c) Huang, Y.-S.; Wang, J.; Zheng, W.-X.; Zhang, F.-L.; Yu, Y.-J.; Zheng, M.; Zhou, X.; Wang, Y.-F. Regioselective radical hydroboration of electron-deficient alkenes: synthesis of α-boryl functionalized molecules. Chem. Commun. 2019, 55, 11904–11907;
(d) Shimoi, M.; Watanabe, T.; Maeda, K.; Curran, D. P.; Taniguchi, T. Radical trans-Hydroboration of Alkynes with N-Heterocyclic Carbene Boranes. Angew. Chem. Int. Ed. 2018, 130, 9629–9634;
10.1002/ange.201804515 Google Scholar(e) Li, G.; Huang, G.; Sun, R.; Curran, D.; Dai, W. Regioselective Radical Borylation of α,β-Unsaturated Esters and Related Compounds by Visible Light Irradiation with an Organic Photocatalyst. Org. Lett. 2021, 23, 4353–4357; (f) Liu, Y.; Li, J.-L.; Liu, X.-G.; Wu, J.-Q.; Huang, Z.-S.; Li, Q.; Wang, H. Radical borylative cyclization of isocyanoarenes with N-heterocyclic carbene borane: synthesis of borylated aza-arenes. Org. Lett. 2021, 23, 1891–1897; (g) Liu, X.; Lin, E. E.; Chen, G.; Li, J.-L.; Liu, P.; Wang, H. Radical Hydroboration and Hydrosilylation of Gem-Difluoroalkenes: Synthesis of α-Difluorinated Alkylborons and Alkylsilanes. Org. Lett. 2019, 21, 8454–8458; (h) Wang, K.; Zhuang, Z.; Tia, H.; Wu, P.; Zhao, X.; Wang, H. Et2Zn-promoted β-trans-selective hydroboration of ynamide. Chin. Chem. Lett. 2020, 31, 1564–1567.
- 19(a) Wang, Y.; Zheng, H.; Xu, J.; Zhuang, C.; Liu, X.; Cao, H. Access to diverse primary, secondary, and tertiary amines via the merger of controllable cleavage of triazines and site-selective functionalization. Org. Chem. Front. 2021, 8, 4706–4714; (b) Lei, S.; Chen, G.; Mai, Y.; Chen, L.; Cai, H.; Tan, J.; Cao, H. Regioselective Copper-Catalyzed Oxidative Cross-Coupling of Imidazo [1,2-a] pyridines with Methyl Ketones: An Efficient Route for Synthesis of 1,2-Diketones. Adv. Synth. Catal. 2016, 358, 67–73; (c) Lei, S.; Mai, Y.; Yan, C.; Mao, J.; Cao, H. A Carbonylation Approach Toward Activation of C-sp2-H and C-sp3-H Bonds: Cu-Catalyzed Regioselective Cross Coupling of Imidazo [1,2-a] pyridines with Methyl Hetarenes. Org. Lett. 2016, 18, 3582–3585; (d) Cui, Z.; Zhu, B.; Li, X.; Cao, H. Access to sulfonylated furans or imidazo [1,2-a] pyridines via a metal-free three-component, domino reaction. Org. Chem. Front. 2018, 5, 2219–2223.




