Convenient Synthesis of Thioester-Substituted Oxindoles by Palladium-Catalyzed Thiocarbonylative Cyclization with Sulfonyl Chlorides as the Sulfur Source
Ren-Rui Xu
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
Search for more papers by this authorXiuyu Fang
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
Search for more papers by this authorCorresponding Author
Xinxin Qi
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiao-Feng Wu
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Leibniz-Institut für Katalyse e.V., Albert-Einstein-Straße 29a, Rostock, 18059 Germany
E-mail: [email protected]; [email protected]Search for more papers by this authorRen-Rui Xu
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
Search for more papers by this authorXiuyu Fang
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
Search for more papers by this authorCorresponding Author
Xinxin Qi
Department of Chemistry, Key Laboratory of Surface & Interface Science of Polymer Materials of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiao-Feng Wu
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Leibniz-Institut für Katalyse e.V., Albert-Einstein-Straße 29a, Rostock, 18059 Germany
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
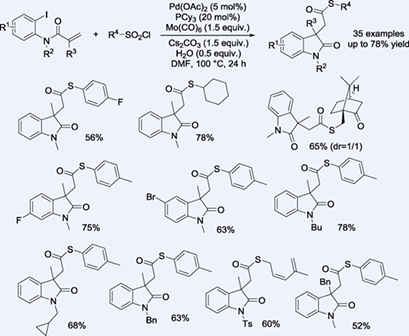
A general and straightforward strategy for the synthesis of thioester-substituted oxindoles via a palladium-catalyzed thiocarbonylative cyclization process has been developed. With sulfonyl chlorides as promising sulfur source, a wide range of thioester-substituted oxindoles were obtained in moderate to high yields. Both aryl and alkyl sulfonyl chlorides were well tolerated, and Mo(CO)6 played a dual role as both a CO source and a reductant in this approach.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200530-sup-0001-Supinfo.pdfPDF document, 6.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Khetmalis, Y. M.; Shivani, M.; Murugesan, S.; Chandra Sekhar, K. V. G. Oxindole and its derivatives: A review on recent progress in biological activities. Biomed. Pharmacother. 2021, 141, 111842; (b) Dhokne, P.; Sakla, A. P.; Shankaraiah, N. Structural insights of oxindole based kinase inhibitors as anticancer agents: Recent advances. Eur. J. Med. Chem. 2021, 216, 113334; (c) Saraswat, P.; Jeyabalan, G.; Hassan, M. Z.; Rahman, M. U.; Nyola, N. K. Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moieties. Synth. Commun. 2016, 46, 1643–1664; (d) Kaur, M.; Singh, M.; Chadha, N.; Silakari, O. Oxindole: A chemical prism carrying plethora of therapeutic benefits. Eur. J. Med. Chem. 2016, 123, 858–894; (e) Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. Therapeutic Potential of Spirooxindoles as Antiviral Agents. ACS Infect. Dis. 2016, 2, 382–392.
- 2(a) Ziarani, G. M.; Javadi, F.; Mohajer, F. The Molecular Diversity Scope of Oxindole Derivatives in Organic Synthesis. Curr. Org. Chem. 2021, 25, 779–818; (b) Sakla, A. P.; Kansal, P.; Shankaraiah, N. Syntheses and Applications of Spirocyclopropyl Oxindoles: A Decade Review. Eur. J. Org. Chem. 2021, 2021, 757–772; (c) Cao, Z.-Y.; Zhou, F.; Zhou, J. Development of Synthetic Methodologies via Catalytic Enantioselective Synthesis of 3,3-Disubstituted Oxindoles. Acc. Chem. Res. 2018, 51, 1443–1454; (d) Liu, Y.; Wang, H.; Wan, J. Recent Advances in Diversity Oriented Synthesis through Isatin-based Multicomponent Reactions. Asian J. Org. Chem. 2013, 2, 374–386; (e) Lin, J.; Jia, M.; Ma, S. Pd-Catalyzed 2,3-Allenylation of Oxindoles with 2,3-Allenylic Carbonates. Chin. J. Chem. 2021, 39, 3044–3050; (f) Simur, T. T.; Dagnaw, F. W.; Yu, Y.-J.; Zhang, F.-L.; Wang, Y.-F. 4-Dimethylaminopyridine-Boryl Radical Promoted Monodefluorinative Alkylation of 3,3-Difluorooxindoles. Chin. J. Chem. 2022, 40, 577–581.
- 3For selected reviews, see: (a) Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of Quaternary Stereocenters by Palladium-Catalyzed Carbopalladation-Initiated Cascade Reactions. Angew. Chem. Int. Ed. 2019, 58, 1562–1573; (b) Marchese, A. D.; Larin, E. M.; Mirabi, B.; Lautens, M. Metal-Catalyzed Approaches toward the Oxindole Core. Acc. Chem. Res. 2020, 53, 1605–1619; (c) Reznikov, A. N.; Ashatkina, M. A.; Klimochkin, Y. N. Recent Developments in Asymmetric Heck Type Cyclization Reactions for Constructions of Complex Molecules. Org. Biomol. Chem. 2021, 19, 5673–5701.
- 4For selected reviews and reports, see: (a) Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133;
(b) Wu, X.-F.; Neumann, H.; Beller, M. Critical Review Palladium-Catalyzed Carbonylative Coupling Reactions between Ar-X and Carbon Nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009;
(c) Wu, X.-F.; Neumann, H. Ruthenium and Rhodium-Catalyzed Carbonylation Reactions. ChemCatChem 2012, 4, 447–458;
(d) Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative Carbonylation as a Powerful Tool for the Direct Synthesis of Carbonylated Heterocycles. Eur. J. Org. Chem. 2012, 2012, 6825–6839;
(e) Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35;
(f) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Carbonylation Reactions of Alkyl Iodides through the Interplay of Carbon Radicals and Pd Catalysts. Acc. Chem. Res. 2014, 47, 1563–1574;
(g) Wu, X.-F. Palladium-catalyzed Carbonylative Transformation of Aryl Chlorides and Aryl Tosylates. RSC Adv. 2016, 6, 83831–83837;
(h) Bai, Y.; Davis, D. C.; Dai, M. Natural Product Synthesis via Palladium-Catalyzed Carbonylation. J. Org. Chem. 2017, 82, 2319–2328;
(i) Peng, J.-B.; Wu, F.-P.; Wu, X.-F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127;
(j) Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526−552;
(k) Gabriele, B. Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry, Wiley, 2021;
10.1002/9783527829354 Google Scholar(l) Zhao, F.; Ai, H.-J.; Wu, X.-F. Radical Carbonylation under Low CO Pressure: Synthesis of Esters from Activated Alkylamines at Transition Metal-Free Conditions. Chin. J. Chem. 2021, 39, 927–932; (m) Shi, L.; Wen, M.; Li, F. Palladium-Catalyzed Tandem Carbonylative Aza-Wacker-Type Cyclization of Nucleophile Tethered Alkene to Access Fused N-Heterocycles. Chin. J. Chem. 2021, 39, 317–322.
- 5For selected reports, see: (a) Cheng, C.; Xiang, J.-N.; Zhu, Y.-P.; Peng, Z.-H.; Li, J.-H. Nickel-Catalyzed Arylcarbamoylation of Alkenes of N-(o-Iodoaryl)acrylamides with Nitroarenes via Reductive Aminocarbonylation: Facile Synthesis of Carbamoyl-Substituted Oxindoles. Org. Lett. 2021, 23, 9543–9547; (b) Chen, C.; Liu, L.; Sun, W.; Zhu, B. Palladium-Catalyzed Aryl-Carbamoylation of Alkene-Tethered Carbamoyl Chlorides: Access to Diverse Aryl-Functionalized Oxindoles. ChemistrySelect 2021, 6, 6464–6467; (c) Feng, Z.; Li, Q.; Chen, L.; Yao, H.; Lin, A. Palladium-catalyzed asymmetric carbamoyl-carbonylation of alkenes. Sci. China Chem. 2021, 64, 1367–1371; (d) Feng, Y.; Yang, S.; Zhao, S.; Zhang, D.-P.; Li, X.; Liu, H.; Dong, Y.; Sun, F.-G. Nickel-Catalyzed Reductive Aryl Thiocarbonylation of Alkene via Thioester Group Transfer Strategy. Org. Lett. 2020, 22, 6734–6738; (e) Chen, M.; Yang, X. P.; Kou, X.; Ren, Z.-H.; Guan, Z.-H. Palladium-Catalyzed Enantioselective Heck Carbonylation with a Monodentate Phosphoramidite Ligand: Asymmetric Synthesis of (+)-Physostigmine, (+)-Physovenine, and (+)-Folicanthine. Angew. Chem. Int. Ed. 2020, 59, 12199–12205; (f) Hu, H.; Teng, F.; Liu, J.; Hu, W.; Luo, S.; Zhu, Q. Enantioselective Synthesis of 2-Oxindole Spirofused Lactones and Lactams by Heck/Carbonylative Cyclization Sequences: Method Development and Applications. Angew. Chem. Int. Ed. 2019, 58, 9225–9229; (g) Liu, X.; Gu, Z. Pd-catalyzed Heck cyclization and in situ hydrocarboxylation or hydromethenylation via a hydrogen borrowing strategy. Org. Chem. Front. 2015, 2, 778–782; (h) Liu, X.; Li, B.; Gu, Z. Palladium-Catalyzed Heck-type Domino Cyclization and Carboxylation to Synthesize Carboxylic Acids by Utilizing Chloroform as the Carbon Monoxide Source. J. Org. Chem. 2015, 80, 7547–7554; (i) Seashore-Ludlow, B.; Danielsson, J.; Somfai, P. Domino Carbopalladation-Carbonylation: Investigation of Substrate Scope. Adv. Synth. Catal. 2012, 354, 205–216; (j) Gabriele, B.; Salerno, G.; Veltri, L.; Costa, M.; Massera, C. Stereoselective Synthesis of (E)-3-(Methoxycarbonyl)methylene-1,3-dihydroindol-2-ones by Palladium-Catalyzed Oxidative Carbonylation of 2-Ethynylanilines. Eur. J. Org. Chem. 2001, 4607–4613.
- 6(a) llardi, E. A.; Vitaku, E.; Njardarson, J. T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842; (b) Parthasarathy, A.; Buckel, W.; Smith, D. M. On the thermodynamic equilibrium between (R)-2-hydroxyacyl-CoA and 2-enoyl-CoA. FEBS J. 2010, 277, 1738–1746; (c) Li, Z.; Dilger, J. M.; Pejaver, V.; Smiley, D.; Arnold, R. J.; Mooney, S. D.; Mukhopadhyay, S.; Radivojac, P.; Clemmer, D. E. Intrinsic size parameters for palmitoylated and carboxyamidomethylated peptides. Int. J. Mass Spectrom. 2014, 368, 6–14; (d) Liu, X.-L.; Shi, Y.; Kang, J. S.; Oelschlaeger, P.; Yang, K.-W. Amino acid thioester derivatives: a highly promising scaffold for the development of metallo-β-lactamase L1 inhibitors. ACS Med. Chem. Lett. 2015, 6, 660–664.
- 7(a) Miyazaki, T.; Han-ya, Y.; Tokuyama, H.; Fukuyama, T. New odorless protocols for the synthesis of aldehydes and ketones from thiol esters. Synlett 2004, 3, 477–480; (b) Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Lin, S.-C.; Li, L.; Fukuyama, T. Facile palladium-mediated conversion of ethanethiol esters to aldehydes and ketones. J. Braz. Chem. Soc. 1998, 9, 381–387; (c) Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Fukuyama, T. A novel ketone synthesis by a palladium-catalyzed reaction of thiol esters and organozinc reagents. Tetrahedron Lett. 1998, 39, 3189–3192; (d) Liebeskind, L. S.; Srogl, J. Thiol ester-boronic acid coupling. A mechanistically unprecedented and general ketone synthesis. J. Am. Chem. Soc. 2000, 122, 11260–11261; (e) Fausett, B. W.; Liebeskind, L. S. Palladium-catalyzed coupling of thiol esters with aryl and primary and secondary alkyl organoindium reagents. J. Org. Chem. 2005, 70, 4851–4853; (f) Wittenberg, R.; Srogl, J.; Egi, M.; Liebeskind, L. S. Ketone synthesis under neutral conditions. Cu(I) diphenylphosphinate-mediated, palladium- catalyzed coupling of thiol esters and organostannanes. Org. Lett. 2003, 5, 3033–3035; (g) Villalobos, J. M.; Srogl, J.; Liebeskind, L. S. A new paradigm for carbon-carbon bond formation: Aerobic, copper- templated cross-coupling. J. Am. Chem. Soc. 2007, 129, 15734–15735; (h) Os'kina, I. A.; Vlasov, V. M. Activation parameters of the reactions of 4-nitrophenyl benzoates and S-phenyl benzothioate with 4-chlorophenol in dimethylformamide in the presence of potassium carbonate. Russ. J. Org. Chem. 2009, 45, 523–527; (i) Ueda, M.; Seki, K.; Imai, Y. S-and N-acyl derivatives of 2-mercaptobenzoxazole; new, highly reactive acylating agents for synthesis of amides and esters. Synthesis 1981, 991–993; (j) Burhardt, M. N.; Taaning, R. H.; Skrydstrup, T. Pd-catalyzed thiocarbonylation with stoichiometric carbon monoxide: Scope and applications. Org. Lett. 2013, 15, 948–951; (k) Sun, F.; Li, M.; He, C.; Wang, B.; Li, B.; Sui, X.; Gu, Z. Cleavage of the C(O)−S bond of thioesters by palladium/norbornene/copper cooperative catalysis: An efficient synthesis of 2-(arylthio)aryl ketones. J. Am. Chem. Soc. 2016, 138, 7456–7459.
- 8 Dubois, M. R. Catalytic applications of transition-metal complexes with sulfide ligands. Chem. Rev. 1989, 89, 1–9.
- 9(a) Qi, X.; Bao, Z.-P.; Wu, X.-F. Palladium-catalyzed carbonylative transformation of aryl iodides and sulfonyl chlorides: convenient access to thioesters. Org. Chem. Front. 2020, 7, 885–889; (b) Qi, X.; Bao, Z.-P.; Yao, X.-T.; Wu, X.-F. Nickel-Catalyzed Thiocarbonylation of Arylboronic Acids with Sulfonyl Chlorides for the Synthesis of Thioesters. Org. Lett. 2020, 22, 6671–6676; (c) Wang, W.; Qi, X.; Wu, X.-F. Palladium-Catalyzed Thiocarbonylation of Benzyl Chlorides with Sulfonyl Chlorides for the synthesis of Arylacetyl Thioesters. Adv. Synth. Catal. 2021, 363, 2541–2545; (d) Wang, W.; Bao, Z.-P.; Qi, X.; Wu, X.-F. Nickel-Catalyzed One-Pot Carbonylative Synthesis of 2-Mono- and 2,3-Disubstituted Thiochromenones from 2-Bromobenzenesulfonyl Chlorides and Alkynes. Org. Lett. 2021, 23, 6589–6593; (e) Xu, R.-R. Wang, W.; Qi, X.; Wu, X.-F. Palladium-catalyzed cascade Heck-type thiocarbonylation for the synthesis of functionalized thioesters. Org. Chem. Front. 2022, 9, 1417–1421; (f) Huo, Y.-W.; Qi, X.; Wu, X.-F. Nickel-Catalyzed Carbonylative Synthesis of α,β-Unsaturated Thioesters from Vinyl Triflates and Arylsulfonyl Chlorides. Org. Lett. 2022, 24, 4009–4013.




