Stretchable Electrochemical Sensors: From Electrode Fabrication to Cell Mechanotransduction Monitoring†
Wen-Ting Fan
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei-Hua Huang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yan-Ling Liu
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWen-Ting Fan
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei-Hua Huang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yan-Ling Liu
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this author‡Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Comprehensive Summary
Electrochemical sensing faces huge challenges in characterizing the transient release of biochemical molecules from deformed cells, due to the severely mechanical mismatch between rigid electrodes and soft cells. In recent years, the emergence of stretchable electrochemical sensors has made a breakthrough by complying with the deformation of living cells and simultaneous monitoring of mechanically evoked biochemical signals. This review first summarizes two fundamental strategies for the fabrication of stretchable electrodes from the points of structure and material. Next, recent progresses in construction of functionalized interface to improve the performance of stretchable electrochemical sensors are presented. Then, the application of stretchable electrochemical sensors in real-time monitoring of biomolecules released by mechanically sensitive cells is introduced. Finally, some perspectives and challenges of stretchable electrochemical sensors regarding cell detection are discussed.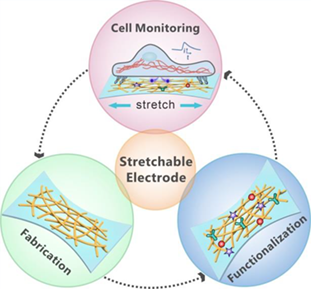
What is the favorite and original chemistry developed in your research group?
The design and construction of electrochemical sensing platform for real-time monitoring of life activities.
What is the most important personality for scientific research?
Innovative spirit, strong curiosity and persistence.
How do you supervise your students?
Encourage students to think much and eager to innovate.
What are your hobbies?
I like music and playing badminton.
Who influences you mostly in your life?
My family and collaborators.
Could you please give us some advices on improving Chinese Journal of Chemistry?
Focusing on the originality of the manuscript.
References
- 1 Wightman, R. M. Probing Cellular Chemistry in Biological Systems with Microelectrodes. Science 2006, 311, 1570–1574.
- 2 Amatore, C.; Arbault, S.; Guille, M.; Lemaitre, F. Electrochemical Monitoring of Single Cell Secretion: Vesicular Exocytosis and Oxidative Stress. Chem. Rev. 2008, 108, 2585–2621.
- 3 Zhang, M.; Yu, P.; Mao, L. Rational Design of Surface/Interface Chemistry for Quantitative in vivo Monitoring of Brain Chemistry. Acc. Chem. Res. 2012, 45, 533–543.
- 4 Phan, N. T. N.; Li, X.; Ewing, A. G. Measuring Synaptic Vesicles Using Cellular Electrochemistry and Nanoscale Molecular Imaging. Nat. Rev. Chem. 2017, 1, 0048.
- 5 Zhang, L.; Tian, Y. Designing Recognition Molecules and Tailoring Functional Surfaces for in vivo Monitoring of Small Molecules in the Brain. Acc. Chem. Res. 2018, 51, 688–696.
- 6 Yu, R. J.; Ying, Y. L.; Gao, R.; Long, Y. T. Confined Nanopipette Sensing: From Single Molecules, Single Nanoparticles, to Single Cells. Angew. Chem. Int. Ed. 2019, 58, 3706–3714.
- 7
Qi, Y. T.; Zhang, F. L.; Tian, S. Y.; Yang, X. K.; Liu, Y. L.; Huang, W. H. Construction of Micro-Nano Electrochemical Sensing Interfaces for Real-Time Monitoring of Single Cells. Sci. Sin. Chim. 2020, 51, 359–373.
10.1360/SSC-2020-0206 Google Scholar
- 8 Turner, A. P. F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196.
- 9 Labib, M.; Sargent, E. H.; Kelley, S. O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090.
- 10 Zhao, W.; Xu, J. J. Chemical Measurement and Analysis: from Phenomenon to Essence. Chin. J. Chem. 2022, 40, 1975–1986.
- 11 Andreescu, S.; Sadik, O. A. Advanced Electrochemical Sensors for Cell Cancer Monitoring. Methods 2005, 37, 84–93.
- 12 Zhou, H.; Du, X.; Zhang, Z. Electrochemical Sensors for Detection of Markers on Tumor Cells. Int. J. Mol. Sci. 2021, 22, 8184.
- 13 Suhito, I. R.; Koo, K. M.; Kim, T. H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2020, 9, 15.
- 14 Santbergen, M. J. C.; van der Zande, M.; Bouwmeester, H.; Nielen, M. W. F. Online and in situ Analysis of Organs-on-a-Chip. Trends Anal. Chem. 2019, 115, 138–146.
- 15 Tian, C.; Tu, Q.; Liu, W.; Wang, J. Recent Advances in Microfluidic Technologies for Organ-on-a-Chip. Trends Anal. Chem. 2019, 117, 146–156.
- 16 Aleman, J.; Kilic, T.; Mille, L. S.; Shin, S. R.; Zhang, Y. S. Microfluidic Integration of Regeneratable Electrochemical Affinity-Based Biosensors for Continual Monitoring of Organ-on-a-Chip devices. Nat. Protoc. 2021, 16, 2564–2593.
- 17 Hochstetler, S. E.; Puopolo, M.; Gustincich, S.; Raviola, E.; Wightman, R. M. Real-Time Amperometric Measurements of Zeptomole Quantities of Dopamine Released from Neurons. Anal. Chem. 2000, 72, 489–496.
- 18 Staal, R. G.; Mosharov, E. V.; Sulzer, D. Dopamine Neurons Release Transmitter via a Flickering Fusion Pore. Nat. Neurosci. 2004, 7, 341–346.
- 19 Wu, W. Z.; Huang, W. H.; Wang, W.; Wang, Z. L.; Cheng, J. K.; Xu, T.; Zhang, R. Y.; Chen, Y.; Liu, J. Monitoring Dopamine Release from Single Living Vesicles with Nanoelectrodes. J. Am. Chem. Soc. 2005, 127, 8914–8915.
- 20 Wang, W.; Zhang, S. H.; Li, L. M.; Wang, Z. L.; Cheng, J. K.; Huang, W. H. Monitoring of Vesicular Exocytosis from Single Cells Using Micrometer and Nanometer-Sized Electrochemical Sensors. Anal. Bioanal. Chem. 2009, 394, 17–32.
- 21 Liu, J. T.; Hu, L. S.; Liu, Y. L.; Chen, R. S.; Cheng, Z.; Chen, S. J.; Amatore, C.; Huang, W. H.; Huo, K. F. Real-Time Monitoring of Auxin Vesicular Exocytotic Efflux from Single Plant Protoplasts by Amperometry at Microelectrodes Decorated with Nanowires. Angew. Chem. Int. Ed. 2014, 53, 2643–2647.
- 22 Clausmeyer, J.; Schuhmann, W. Nanoelectrodes: Applications in Electrocatalysis, Single-Cell Analysis and High-Resolution Electrochemical Imaging. Trends Anal. Chem. 2016, 79, 46–59.
- 23 Wang, Y.; Noel, J. M.; Velmurugan, J.; Nogala, W.; Mirkin, M. V.; Lu, C.; Guille Collignon, M.; Lemaitre, F.; Amatore, C. Nanoelectrodes for Determination of Reactive Oxygen and Nitrogen Species inside Murine Macrophages. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 11534–11539.
- 24 Li, X.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A. G. Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes. Angew. Chem. Int. Ed. 2015, 54, 11978–11982.
- 25 Li, Y. T.; Zhang, S. H.; Wang, L.; Xiao, R. R.; Liu, W.; Zhang, X. W.; Zhou, Z.; Amatore, C.; Huang, W. H. Nanoelectrode for Amperometric Monitoring of Individual Vesicular Exocytosis inside Single Synapses. Angew. Chem. Int. Ed. 2014, 53, 12456–12460.
- 26 Zhang, X. W.; Qiu, Q. F.; Jiang, H.; Zhang, F. L.; Liu, Y. L.; Amatore, C.; Huang, W. H. Real-Time Intracellular Measurements of ROS and RNS in Living Cells with Single Core-Shell Nanowire Electrodes. Angew. Chem. Int. Ed. 2017, 56, 12997–13000.
- 27 Qi, Y. T.; Jiang, H.; Wu, W. T.; Zhang, F. L.; Tian, S. Y.; Fan, W. T.; Liu, Y. L.; Amatore, C.; Huang, W. H. Homeostasis inside Single Activated Phagolysosomes: Quantitative and Selective Measurements of Submillisecond Dynamics of Reactive Oxygen and Nitrogen Species Production with a Nanoelectrochemical Sensor. J. Am. Chem. Soc. 2022, 144, 9723–9733.
- 28 Zhang, F. L.; Tang, Y.; Jiang, H.; Yang, X. K.; Huang, W. H. Harpagide Inhibits Microglial Activation and Protects Dopaminergic Neurons as Revealed by Nanoelectrode Amperometry. Chin. J. Chem. 2021, 39, 2188–2194.
- 29 Wang, N.; Tytell, J. D.; Ingber, D. E. Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82.
- 30 Hoffman, B. D.; Grashoff, C.; Schwartz, M. A. Dynamic Molecular Processes Mediate Cellular Mechanotransduction. Nature 2011, 475, 316–323.
- 31 Humphrey, J. D.; Dufresne, E. R.; Schwartz, M. A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812.
- 32 Murthy, S. E.; Dubin, A. E.; Patapoutian, A. Piezos Thrive under Pressure: Mechanically Activated Ion Channels in Health and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783
- 33 Hu, R.; Guille, M.; Arbault, S.; Lin, C. J.; Amatore, C. In Situ Electrochemical Monitoring of Reactive Oxygen and Nitrogen Species Released by Single Mg63 Osteosarcoma Cell Submitted to a Mechanical Stress. Phys. Chem. Chem. Phys. 2010, 12, 10048–10054.
- 34 Hecht, E.; Liedert, A.; Ignatius, A.; Mizaikoff, B.; Kranz, C. Local Detection of Mechanically Induced ATP Release from Bone Cells with ATP Microbiosensors. Biosens. Bioelectron. 2013, 44, 27–33.
- 35 Morris, R.; Fagan-Murphy, A.; MacEachern, S. J.; Covill, D.; Patel, B. A. Electrochemical Fecal Pellet Sensor for Simultaneous Real-Time ex Vivo Detection of Colonic Serotonin Signalling and Motility. Sci. Rep. 2016, 6, 23442.
- 36 Forrest, S. R. The Path to Ubiquitous and Low-Cost Organic Electronic Appliances on Plastic. Nature 2004, 428, 911–918.
- 37 Hammock, M. L.; Chortos, A.; Tee, B. C.; Tok, J. B.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038.
- 38 Cheng, T.; Zhang, Y.; Lai, W. Y.; Huang, W. Stretchable Thin-Film Electrodes for Flexible Electronics with High Deformability and Stretchability. Adv. Mater. 2015, 27, 3349–3376.
- 39 Yao, S.; Zhu, Y. Nanomaterial-Enabled Stretchable Conductors: Strategies, Materials and Devices. Adv. Mater. 2015, 27, 1480–1511.
- 40 Mackanic, D. G.; Chang, T. H.; Huang, Z.; Cui, Y.; Bao, Z. Stretchable Electrochemical Energy Storage Devices. Chem. Soc. Rev. 2020, 49, 4466–4495.
- 41 Wang, S.; Oh, J. Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045.
- 42 Ray, T. R.; Choi, J.; Bandodkar, A. J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J. A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533.
- 43 Yang, J. C.; Mun, J.; Kwon, S. Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765.
- 44 Trung, T. Q.; Lee, N. E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoring and Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372.
- 45 Kim, J.; Campbell, A. S.; de Ávila, B. E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- 46 Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491.
- 47 Sempionatto, J. R.; Lin, M.; Yin, L.; De la paz, E.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A. N.; Khorshed, A. A.; Zhang, F.; Tostado, N.; Xu, S.; Wang, J. An Epidermal Patch for the Simultaneous Monitoring of Haemodynamic and Metabolic Biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748.
- 48 Liu, Y. L.; Huang, W. H. Stretchable Electrochemical Sensors for Cell and Tissue Detection. Angew. Chem. Int. Ed. 2021, 60, 2757–2767.
- 49 Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; Huang, C.; Sheng, X.; Zhang, M.; Yin, L. A Flexible and Physically Transient Electrochemical Sensor for Real-Time Wireless Nitric Oxide Monitoring. Nat. Commun. 2020, 11, 3207.
- 50 Deng, Y.; Qi, H.; Ma, Y.; Liu, S.; Zhao, M.; Guo, Z.; Jie, Y.; Zheng, R.; Jing, J.; Chen, K.; Ding, H.; Lv, G.; Zhang, K.; Li, R.; Cheng, H.; Zhao, L.; Sheng, X.; Zhang, M.; Yin, L. A Flexible and Highly Sensitive Organic Electrochemical Transistor-Based Biosensor for Continuous and Wireless Nitric Oxide Detection. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2208060119.
- 51 Rogers, J. A.; Someya, T.; Huang, Y. G. Materials and Mechanics for Stretchable Electronics. Science 2010, 327, 1603–1607.
- 52 Zhao, S.; Li, J.; Cao, D.; Zhang, G.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.; Sun, R.; Wong, C. P. Recent Advancements in Flexible and Stretchable Electrodes for Electromechanical Sensors: Strategies, Materials, and Features. ACS Appl. Mater. Interfaces 2017, 9, 12147–12164.
- 53 Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and Structural Designs of Stretchable Conductors. Chem. Soc. Rev. 2019, 48, 2946–2966.
- 54
Ma, Y.; Li, H.; Chen, S.; Liu, Y.; Meng, Y.; Cheng, J.; Feng, X. Skin-Like Electronics for Perception and Interaction: Materials, Structural Designs, and Applications. Adv. Intell. Syst. 2020, 3, 2000108.
10.1002/aisy.202000108 Google Scholar
- 55 Yao, S.; Ren, P.; Song, R.; Liu, Y.; Huang, Q.; Dong, J.; O'Connor, B. T.; Zhu, Y. Nanomaterial-Enabled Flexible and Stretchable Sensing Systems: Processing, Integration, and Applications. Adv. Mater. 2020, 32, 1902343.
- 56 Kim, D. H.; Rogers, J. A. Stretchable Electronics: Materials Strategies and Devices. Adv. Mater. 2008, 20, 4887–4892.
- 57 Liu, Y.; He, K.; Chen, G.; Leow, W. R.; Chen, X. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev. 2017, 117, 12893–12941.
- 58 Cai, P.; Wang, C.; Gao, H.; Chen, X. Mechanomaterials: A Rational Deployment of Forces and Geometries in Programming Functional Materials. Adv. Mater. 2021, 33, 2007977.
- 59 Bandodkar, A. J.; Nunez-Flores, R.; Jia, W.; Wang, J. All-Printed Stretchable Electrochemical Devices. Adv. Mater. 2015, 27, 3060–3065.
- 60 Lee, H.; Song, C.; Hong, Y. S.; Kim, M.; Cho, H. R.; Kang, T.; Shin, K.; Choi, S. H.; Hyeon, T.; Kim, D. H. Wearable/Disposable Sweat-Based Glucose Monitoring Device with Multistage Transdermal Drug Delivery Module. Sci. Adv. 2017, 3, e1601314.
- 61 Fan, J. A.; Yeo, W. H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S. Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; Bajema, M.; Coleman, T.; Gregoire, D.; Larsen, R. J.; Huang, Y.; Rogers, J. A. Fractal Design Concepts for Stretchable Electronics. Nat. Commun. 2014, 5, 3266.
- 62 Sim, K.; Li, Y.; Song, J.; Yu, C. Biaxially Stretchable Ultrathin Si Enabled by Serpentine Structures on Prestrained Elastomers. Adv. Mater. Technol. 2019, 4, 1800489.
- 63 Gonzalez, M.; Axisa, F.; Bulcke, M. V.; Brosteaux, D.; Vandevelde, B.; Vanfleteren, J. Design of Metal Interconnects for Stretchable Electronic Circuits. Microelectron. Reliab. 2008, 48, 825–832.
- 64 Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C. F. Flexible Electronics: Stretchable Electrodes and Their Future. Adv. Funct. Mater. 2019, 29, 1805924.
- 65 Guo, C. F.; Liu, Q.; Wang, G.; Wang, Y.; Shi, Z.; Suo, Z.; Chu, C. W.; Ren, Z. Fatigue-Free, Superstretchable, Transparent, and Biocompatible Metal Electrodes. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 12332–12337.
- 66 Lee, S.; Sasaki, D.; Kim, D.; Mori, M.; Yokota, T.; Lee, H.; Park, S.; Fukuda, K.; Sekino, M.; Matsuhisa, K.; Shimizu, T.; Someya, T. Ultrasoft Electronics to Monitor Dynamically Pulsing Cardiomyocytes. Nat. Nanotechnol. 2019, 14, 156–160.
- 67 Yan, L. P.; Wen, M. Y.; Qin, Y.; Bi, C. X.; Zhao, Y.; Fan, W. T.; Yan, J.; Huang, W. H.; Liu, Y. L. Soft Electrodes for Electrochemical and Electrophysiological Monitoring of Beating Cardiomyocytes. Angew. Chem. Int. Ed. 2022, 61, e202203757.
- 68Jiang, H; Khang, D. Y.; Song, J.; Sun, Y.; Huang, Y.; Rogers, J. A. Finite Deformation Mechanics in Buckled Thin Films on Compliant Supports. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 15607–15612.
- 69 Qi, D.; Liu, Z.; Yu, M.; Liu, Y.; Tang, Y.; Lv, J.; Li, Y.; Wei, J.; Liedberg, B.; Yu, Z.; Chen, X. Highly Stretchable Gold Nanobelts with Sinusoidal Structures for Recording Electrocorticograms. Adv. Mater. 2015, 27, 3145–3151.
- 70 Zang, J.; Cao, C.; Feng, Y.; Liu, J.; Zhao, X. Stretchable and High-Performance Supercapacitors with Crumpled Graphene Papers. Sci. Rep. 2014, 4, 6492.
- 71 Liu, Z.; Wang, X.; Qi, D.; Xu, C.; Yu, J.; Liu, Y.; Jiang, Y.; Liedberg, B.; Chen, X. High-Adhesion Stretchable Electrodes Based on Nanopile Interlocking. Adv. Mater. 2017, 29, 1603382.
- 72 Chen, X. Making Electrodes Stretchable. Small Methods 2017, 1, 1600029.
- 73 Liu, Z. F.; Fang, S.; Moura, F. A.; Ding, J. N.; Jiang, N.; Di, J.; Zhang, M.; Lepró, X.; Galvão, D. S.; Haines, C. S.; Yuan, N. Y.; Yin, S. G.; Lee, D. W.; Wang, R.; Wang, H. Y.; Lv, W.; Dong, C.; Zhang, R. C.; Chen, M. J.; Yin, Q.; Chong, Y. T.; Zhang, R.; Wang, X.; Lima, M. D.; Ovalle-Robles, R.; Qian, D.; Lu, H.; Baughman, R. H. Hierarchically Buckled Sheath-Core Fibers for Superelastic Electronics, Sensors, and Muscles. Science 2015, 349, 400–404.
- 74 Zhao, X.; Wang, K.; Li, B.; Wang, C.; Ding, Y.; Li, C.; Mao, L.; Lin, Y. Fabrication of a Flexible and Stretchable Nanostructured Gold Electrode Using a Facile Ultraviolet-Irradiation Approach for the Detection of Nitric Oxide Released from Cells. Anal. Chem. 2018, 90, 7158–7163.
- 75 Dickey, M. D. Stretchable and Soft Electronics Using Liquid Metals. Adv. Mater. 2017, 29, 1606425.
- 76 Kazem, N.; Hellebrekers, T.; Majidi, C. Soft Multifunctional Composites and Emulsions with Liquid Metals. Adv. Mater. 2017, 29, 1605985.
- 77 Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in Liquid Metals for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 2518–2533.
- 78 Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L.; Lu, Y.; Jiang, X. Printed Stretchable Liquid Metal Electrode Arrays for in Vivo Neural Recording. Small 2021, 17, 2006612.
- 79 Liu, Y. L.; Jin, Z. H.; Liu, Y. H.; Hu, X. B.; Qin, Y.; Xu, J. Q.; Fan, C. F.; Huang, W. H. Stretchable Electrochemical Sensor for Real-Time Monitoring of Cells and Tissues. Angew. Chem. Int. Ed. 2016, 55, 4537–4541.
- 80 Wang, Y.; Gong, S.; Wang, S. J.; Yang, X.; Ling, Y.; Yap, L. W.; Dong, D.; Simon, G. P.; Cheng, W. Standing Enokitake-Like Nanowire Films for Highly Stretchable Elastronics. ACS Nano 2018, 12, 9742–9749.
- 81 Wang, Y.; Gong, S.; Gomez, D.; Ling, Y.; Yap, L. W.; Simon, G. P.; Cheng, W. Unconventional Janus Properties of Enokitake-Like Gold Nanowire Films. ACS Nano 2018, 12, 8717–8722.
- 82 Zhai, Q.; Wang, Y.; Gong, S.; Ling, Y.; Yap, L. W.; Liu, Y.; Wang, J.; Simon, G. P.; Cheng, W. Vertical Gold Nanowires Stretchable Electrochemical Electrodes. Anal. Chem. 2018, 90, 13498–13505.
- 83 Lyu, Q.; Zhai, Q.; Dyson, J.; Gong, S.; Zhao, Y.; Ling, Y.; Chandrasekaran, R.; Dong, D.; Cheng, W. Real-Time and in-Situ Monitoring of H2O2 Release from Living Cells by a Stretchable Electrochemical Biosensor Based on Vertically Aligned Gold Nanowires. Anal. Chem. 2019, 91, 13521–13527.
- 84 Jin, Z. H.; Liu, Y. L.; Chen, J. J.; Cai, S. L.; Xu, J. Q.; Huang, W. H. Conductive Polymer-Coated Carbon Nanotubes to Construct Stretchable and Transparent Electrochemical Sensors. Anal. Chem. 2017, 89, 2032–2038.
- 85 Kayser, L. V.; Lipomi, D. J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133.
- 86 Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N. I.; Chen, Z.; Chung, J. W.; Linder, C.; Toney, M. F.; Murmann, B.; Bao, Z. A Highly Stretchable, Transparent, and Conductive Polymer. Sci. Adv. 2017, 3, e1602076.
- 87 Moon, G. D.; Lim, G. H.; Song, J. H.; Shin, M.; Yu, T.; Lim, B.; Jeong, U. Highly Stretchable Patterned Gold Electrodes Made of Au Nanosheets. Adv. Mater. 2013, 25, 2707–2712.
- 88 Naguib, M.; Mochalin, V. N.; Barsoum, M. W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005.
- 89 Ling, Z.; Ren, C. E.; Zhao, M. Q.; Yang, J.; Giammarco, J. M.; Qiu, J.; Barsoum, M. W.; Gogotsi, Y. Flexible and Conductive MXene Films and Nanocomposites with High Capacitance. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 16676–16681.
- 90 Anasori, B.; Lukatskaya, M. R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098.
- 91 Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M. T.; Iatsunskyi, I. Mxene Nanoflakes Decorating Zno Tetrapods for Enhanced Performance of Skin-Attachable Stretchable Enzymatic Electrochemical Glucose Sensor. Biosens. Bioelectron. 2022, 207, 114141.
- 92 Guo, C. F.; Sun, T.; Liu, Q.; Suo, Z.; Ren, Z. Highly Stretchable and Transparent Nanomesh Electrodes Made by Grain Boundary Lithography. Nat. Commun. 2014, 5, 3121.
- 93 Shu, Y.; Lu, Q.; Yuan, F.; Tao, Q.; Jin, D.; Yao, H.; Xu, Q.; Hu, X. Stretchable Electrochemical Biosensing Platform Based on Ni-MOF Composite/Au Nanoparticle-Coated Carbon Nanotubes for Real-Time Monitoring of Dopamine Released from Living Cells. ACS Appl. Mater. Interfaces 2020, 12, 49480–49488.
- 94 Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; Zheng, S.; Sun, X.; Xu, F.; Yu, H.; Peng, H. Functionalized Helical Fibre Bundles of Carbon Nanotubes as Electrochemical Sensors for Long-Term in vivo Monitoring of Multiple Disease Biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171.
- 95 Deng, J.; Xu, Y.; He, S.; Chen, P.; Bao, L.; Hu, Y.; Wang, B.; Sun, X.; Peng, H. Preparation of Biomimetic Hierarchically Helical Fiber Actuators from Carbon Nanotubes. Nat. Protoc. 2017, 12, 1349–1358.
- 96 Chen, P.; Xu, Y.; He, S.; Sun, X.; Pan, S.; Deng, J.; Chen, D.; Peng, H. Hierarchically Arranged Helical Fibre Actuators Driven by Solvents and Vapours. Nat. Nanotechnol. 2015, 10, 1077–1083.
- 97 Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, Fully Recyclable, and Malleable Electronic Skin Enabled by Dynamic Covalent Thermoset Nanocomposite. Sci. Adv. 2018, 4, eaaq0508.
- 98 Ren, W.; Sun, Y.; Zhao, D.; Aili, A.; Zhang, S.; Shi, C.; Zhang, J.; Geng, H.; Zhang, J.; Zhang, L.; Xiao, J.; Yang, R. High-Performance Wearable Thermoelectric Generator with Self-Healing, Recycling, and Lego-Like Reconfiguring Capabilities. Sci. Adv. 2021, 7, eabe0586.
- 99
Zhang, G. H.; Zhang, L.; Zhu, Q. H.; Chen, H.; Yuan, W. L.; Fu, J.; Wang, S. L.; He, L.; Tao, G. H. Self-Healable, Malleable, and Flexible Ionic Polyimine as an Environmental Sensor for Portable Exogenous Pollutant Detection. ACS Mater. Lett. 2021, 4, 136–144.
10.1021/acsmaterialslett.1c00687 Google Scholar
- 100 Wang, Y.; Liu, D.; Zhang, Y.; Fan, L.; Ren, Q.; Ma, S.; Zhang, M. Stretchable Temperature-Responsive Multimodal Neuromorphic Electronic Skin with Spontaneous Synaptic Plasticity Recovery. ACS Nano 2022, 16, 8283–8293.
- 101 Liu, Y. L.; Qin, Y.; Jin, Z. H.; Hu, X. B.; Chen, M. M.; Liu, R.; Amatore, C.; Huang, W. H. A Stretchable Electrochemical Sensor for Inducing and Monitoring Cell Mechanotransduction in Real Time. Angew. Chem. Int. Ed. 2017, 56, 9454–9458.
- 102 Fan, W. T.; Qin, Y.; Hu, X. B.; Yan, J.; Wu, W. T.; Liu, Y. L.; Huang, W. H. Stretchable Electrode Based on Au@Pt Nanotube Networks for Real-Time Monitoring of ROS Signaling in Endothelial Mechanotransduction. Anal. Chem. 2020, 92, 15639–15646.
- 103 Zhou, M.; Jiang, Y.; Wang, G.; Wu, W.; Chen, W.; Yu, P.; Lin, Y.; Mao, J.; Mao, L. Single-Atom Ni-N4 Provides a Robust Cellular NO Sensor. Nat. Commun. 2020, 11, 3188.
- 104 Yan, J.; Qin, Y.; Fan, W. T.; Wu, W. T.; Lv, S. W.; Yan, L. P.; Liu, Y. L.; Huang, W. H. Plasticizer and Catalyst Co-Functionalized PEDOT:PSS Enables Stretchable Electrochemical Sensing of Living Cells. Chem. Sci. 2021, 12, 14432–14440.
- 105 Peng, M.; Zhao, X.; Wang, C.; Guan, L.; Li, K.; Gu, C.; Lin, Y. In Situ Observation of Glucose Metabolism Dynamics of Endothelial Cells in Hyperglycemia with a Stretchable Biosensor: Research Tool for Bridging Diabetes and Atherosclerosis. Anal. Chem. 2021, 93, 1043–1049.
- 106 Liu, Y. L.; Liu, R.; Qin, Y.; Qiu, Q. F.; Chen, Z.; Cheng, S. B.; Huang, W. H. Flexible Electrochemical Urea Sensor Based on Surface Molecularly Imprinted Nanotubes for Detection of Human Sweat. Anal. Chem. 2018, 90, 13081–13087.
- 107 Chen, M. M.; Cheng, S. B.; Ji, K.; Gao, J.; Liu, Y. L.; Wen, W.; Zhang, X.; Wang, S.; Huang, W. H. Construction of a Flexible Electrochemiluminescence Platform for Sweat Detection. Chem. Sci. 2019, 10, 6295–6303.
- 108 Fan, W. T.; Zhao, Y.; Wu, W. T.; Qin, Y.; Yan, J.; Liu, Y. L.; Huang, W. H. Redox Homeostasis Alteration in Endothelial Mechanotransduction Monitored by Dual Stretchable Electrochemical Sensors. Anal. Chem. 2022, 94, 7425–7432.
- 109 Qin, Y.; Hu, X. B.; Fan, W. T.; Yan, J.; Cheng, S. B.; Liu, Y. L.; Huang, W. H. A Stretchable Scaffold with Electrochemical Sensing for 3D Culture, Mechanical Loading, and Real-Time Monitoring of Cells. Adv. Sci. 2021, 8, 2003738.
- 110 Wang, Y. W.; Liu, Y. L.; Xu, J. Q.; Qin, Y.; Huang, W. H. Stretchable and Photocatalytically Renewable Electrochemical Sensor Based on Sandwich Nanonetworks for Real-Time Monitoring of Cells. Anal. Chem. 2018, 90, 5977–5981.
- 111 Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244.
- 112 Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535.
- 113 Xu, J. Q.; Liu, Y. L.; Wang, Q.; Duo, H. H.; Zhang, X. W.; Li, Y. T.; Huang, W. H. Photocatalytically Renewable Micro-Electrochemical Sensor for Real-Time Monitoring of Cells. Angew. Chem. Int. Ed. 2015, 54, 14402–14406.
- 114 Liu, Y. L.; Chen, Y.; Fan, W. T.; Cao, P.; Yan, J.; Zhao, X. Z.; Dong, W. G.; Huang, W. H. Mechanical Distension Induces Serotonin Release from Intestine as Revealed by Stretchable Electrochemical Sensing. Angew. Chem. Int. Ed. 2020, 59, 4075–4081.
- 115 Davies, P. F. Flow-Mediated Endothelial Mechanotransduction. Physiol. Rev. 1995, 75, 519–560.
- 116 Lehoux, S.; Castier, Y.; Tedgui, A. Molecular Mechanisms of the Vascular Responses to Haemodynamic Forces. J. Intern. Med. 2006, 259, 381–392.
- 117 Hahn, C.; Schwartz, M. A. Mechanotransduction in Vascular Physiology and Atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62.
- 118 Ling, Y.; Lyu, Q.; Zhai, Q.; Zhu, B.; Gong, S.; Zhang, T.; Dyson, J.; Cheng, W. Design of Stretchable Holey Gold Biosensing Electrode for Real-Time Cell Monitoring. ACS Sens. 2020, 5, 3165–3171.
- 119 Li, J.; Jiang, M.; Su, M.; Tian, L.; Shi, W.; Yu, C. Stretchable and Transparent Electrochemical Sensor Based on Nanostructured Au on Carbon Nanotube Networks for Real-Time Analysis of H2O2 Release from Cells. Anal. Chem. 2021, 93, 6723–6730.
- 120 Davies, P. F.; Tripathi, S. C. Mechanical Stress Mechanisms and the Cell: An Endothelial Paradigm. Cir. Res. 1993, 72, 239–245.
- 121 Chien, S. Mechanotransduction and Endothelial Cell Homeostasis: The Wisdom of the Cell. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1209–H1224.
- 122 Iskratsch, T.; Wolfenson, H.; Sheetz, M. P. Appreciating Force and Shape-the Rise of Mechanotransduction in Cell Biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833.
- 123 Wang, D. L.; Wung, B. S.; Peng, Y. C.; Wang, J. J. Mechanical Strain Increases Endothelin-1 Gene Expression via Protein Kinase C Pathway in Human Endothelial Cells. J. Cell. Physiol. 1995, 163, 400–406.
- 124 Harrison, D. G.; Widder, J.; Grumbach, I.; Chen, W.; Weber, M.; Searles, C. Endothelial Mechanotransduction, Nitric Oxide and Vascular Inflammation. J. Intern. Med. 2006, 259, 351–363.
- 125 Chatterjee, S.; Fisher, A. B. Mechanotransduction: Forces, Sensors, and Redox Signaling. Antioxid. Redox Sign. 2014, 20, 868–871.
- 126 Cai, H.; Harrison, D. G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844.
- 127 Jufri, N. F.; Abidali, M.; Avolio, A.; Baker, M. S. Mechanical Stretch: Physiological and Pathological Implications for Human Vascular Endothelial Cells. Vascular Cell 2015, 7, 8.
- 128 Galougahi, K. K.; Ashley, E. A.; Ali, Z. A. Redox Regulation of Vascular Remodeling. Cell. Mol. Life Sci. 2016, 73, 349–363.
- 129 Meza, C. A.; La Favor, J. D.; Kim, D. H.; Hickner, R. C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Inter. J. Mol. Sci. 2019, 20, 3775.
- 130 Matsushita, H.; Lee, K. h.; Tsao, P. S. Cyclic Strain Induces Reactive Oxygen Species Production via an Endothelial NAD(P)H Oxidase. J. Cell. Biochem. 2001, 81, 99–106.
- 131 Wright, E. M.; Loo, D. D.; Hirayama, B. A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794.
- 132 Wang, Y.; Li, Q.; Liu, F.; Jin, S.; Zhang, Y.; Zhang, T.; Zhu, Y.; Zhou, Y. Transcriptional Activation of Glucose Transporter 1 in Orthodontic Tooth Movement-Associated Mechanical Response. Int. J. Oral Sci. 2018, 10, 27.
- 133 Coste, B.; Mathur, J.; Schmidt, M.; Earley, T. J.; Ranade, S.; Petrus, M. J.; Dubin, A. E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60.
- 134 Huh, D.; Matthews, B. D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H. Y.; Ingber, D. E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668.
- 135 Doryab, A.; Tas, S.; Taskin, M. B.; Yang, L.; Hilgendorff, A.; Groll, J.; Wagner, D. E.; Schmid, O. Evolution of Bioengineered Lung Models: Recent Advances and Challenges in Tissue Mimicry for Studying the Role of Mechanical Forces in Cell Biology. Adv. Funct. Mater. 2019, 29, 1903114.
- 136 Kim, M.; Javed, N. H.; Yu, J. G.; Christofi, F.; Cooke, H. J. Mechanical Stimulation Activates Gαq Signaling Pathways and 5-Hydroxytryptamine Release from Human Carcinoid Bon Cells. J. Clin. Invest. 2001, 108, 1051–1059.
- 137 Kim, M.; Christofi, F. L.; Xue, J.; Robinson, J. M.; Cooke, H. J. Mechanically Evoked 5-Hydroxytryptamine Release Is Mediated by Caveolin-Associated Cholesterol Rich Membrane Domains. Neurogastroenterol. Motil. 2007, 19, 309–317.
- 138 Bellono, N. W.; Bayrer, J. R.; Leitch, D. B.; Castro, J.; Zhang, C.; O'Donnell, T. A.; Brierley, S. M.; Ingraham, H. A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors That Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.
- 139 Stukel, J. M.; Willits, R. K. Mechanotransduction of Neural Cells through Cell–Substrate Interactions. Tissue Eng. Part B Rev. 2016, 22, 173–182.
- 140 Chighizola, M.; Dini, T.; Lenardi, C.; Milani, P.; Podestà, A.; Schulte, C. Mechanotransduction in Neuronal Cell Development and Functioning. Biophys. Rev. 2019, 11, 701–720.
- 141 Marinval, N.; Chew, S. Y. Mechanotransduction Assays for Neural Regeneration Strategies: A Focus on Glial Cells. APL Bioeng. 2021, 5, 021505.
- 142 Jin, Z. H.; Liu, Y. L.; Fan, W. T.; Huang, W. H. Integrating Flexible Electrochemical Sensor into Microfluidic Chip for Simulating and Monitoring Vascular Mechanotransduction. Small 2019, 16, 1903204.
- 143 Varady, N. H.; Grodzinsky, A. J. Osteoarthritis Year in Review 2015: Mechanics. Osteoarthr. Cartil. 2016, 24, 27–35.
- 144 Sanchez-Adams, J.; Leddy, H. A.; McNulty, A. L.; O'Conor, C. J.; Guilak, F. The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 451.
- 145 Pathria, M. N.; Chung, C. B.; Resnick, D. L. Acute and Stress-Related Injuries of Bone and Cartilage: Pertinent Anatomy, Basic Biomechanics, and Imaging Perspective. Radiology 2016, 280, 21–38.
- 146 Chang, S. H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U. I.; Kim-Kaneyama, J. R.; Yanagita, M.; Economides, A.; Canalis, E.; Chen, D.; Tanaka, S.; Saito, T. Excessive Mechanical Loading Promotes Osteoarthritis through the Gremlin-1-NF-κB Pathway. Nat. Commun. 2019, 10, 1442.
- 147 Marcu, K. B.; Otero, M.; Olivotto, E.; Borzi, R. M.; Goldring, M. B. NF-κB Signaling: Multiple Angles to Target OA. Curr. Drug Targets 2010, 11, 599–613.
Citing Literature
15 February, 2023
Pages 443-457




