Coulometric Response of H+-Selective Solid-Contact Ion-Selective Electrodes and Its Application in Flexible Sensors†
Tingting Han
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Search for more papers by this authorTao Song
State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorShiyu Gan
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Search for more papers by this authorCorresponding Author
Dongxue Han
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Guangdong Provincial Key Laboratory of Psychoactive Substances Monitoring and Safety, Anti-Drug Technology Center of Guangdong Province, Guangzhou, Guangdong, 510230 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorJohan Bobacka
Åbo Akademi University, Johan Gadolin Process Chemistry Centre, Laboratory of Molecular Science and Engineering, Henriksgatan 2, FI-20500, Turku/Åbo, Finland
Search for more papers by this authorCorresponding Author
Li Niu
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Ari Ivaska
Åbo Akademi University, Johan Gadolin Process Chemistry Centre, Laboratory of Molecular Science and Engineering, Henriksgatan 2, FI-20500, Turku/Åbo, Finland
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorTingting Han
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Search for more papers by this authorTao Song
State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorShiyu Gan
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Search for more papers by this authorCorresponding Author
Dongxue Han
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
Guangdong Provincial Key Laboratory of Psychoactive Substances Monitoring and Safety, Anti-Drug Technology Center of Guangdong Province, Guangzhou, Guangdong, 510230 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorJohan Bobacka
Åbo Akademi University, Johan Gadolin Process Chemistry Centre, Laboratory of Molecular Science and Engineering, Henriksgatan 2, FI-20500, Turku/Åbo, Finland
Search for more papers by this authorCorresponding Author
Li Niu
Guangzhou Key Laboratory of Sensing Materials & Devices, Center for Advanced Analytical Science, School of Chemistry and Chemical Engineering, c/o School of Civil Engineering, Guangzhou University, Guangzhou, Guangdong, 510006 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Ari Ivaska
Åbo Akademi University, Johan Gadolin Process Chemistry Centre, Laboratory of Molecular Science and Engineering, Henriksgatan 2, FI-20500, Turku/Åbo, Finland
E-mail: [email protected], [email protected], [email protected]Search for more papers by this author† Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Comprehensive Summary
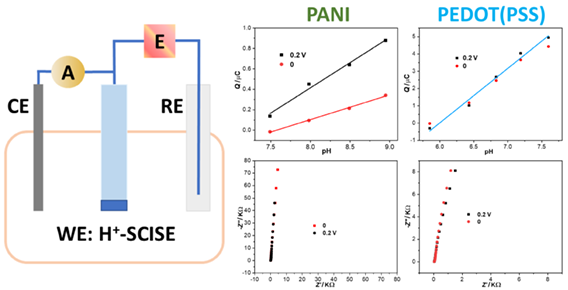
The analytical performance of H+-selective solid-contact ion-selective electrodes (SCISEs) based on solid contact polyaniline doped with chloride (PANI(Cl)) and poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate) (PEDOT(PSS)) was characterized by a developed coulometric signal transduction method. PEDOT(PSS) solid contact is covered by PVC based H+-selective membrane. The obtained coulometric signal demonstrates that the cumulated charge can be amplified by increasing the capacitance of solid contact. SCISEs covered with spin-coated membrane behave faster amperometric response than electrodes with drop-cast membrane. In contrast to earlier works, the amperometric response and impedance spectrum demonstrates H+ transfer through SCISEs is independent from the thickness of membrane. The exceptional behavior of PANI(Cl) H+-SCISEs shows that the capacitance estimated from impedance spectrum at low frequency 10 mHz and coulometric signal of PANI(Cl) based SCISEs is influenced by the applied potentials, whereas PEDOT(PSS) solid contact is independent from the chosen applied potentials. Furthermore, preliminary investigations of coulometric signal transduction on flexible pH sensor implies its potential applications in wearable sensors for sweat ion concentration detection.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200500-sup-0001-Supinfo.pdfPDF document, 375.3 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalysis 2014, 26, 1171-1181.
- 2 Lisak, G. Reliable environmental trace heavy metal analysis with potentiometric ion sensors-reality or a distant dream. Environ. Pollut. 2021, 289, 117882.
- 3 Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128.
- 4 Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351.
- 5 Buhlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688.
- 6 Zhou, M.; Gan, S.; Cai, B.; Li, F.; Ma, W.; Han, D.; Niu, L. Effective solid contact for ion-selective electrodes: tetrakis(4-chlorophenyl) borate (TB-) anions doped nanocluster films. Anal. Chem. 2012, 84, 3480–3483.
- 7 Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937.
- 8 Lindfors, T.; Ivaska, A. Stability of the inner polyaniline solid contact layer in all-solid-state K+-selective electrodes based on plasticized poly(vinyl) chloride. Anal. Chem. 2004, 76, 4387–4394.
- 9 Lindfors, T.; Szucs, J.; Sundfors, F.; Gyursanyi, R. Polyaniline Nanoparticle-Based Solid-Contact Silicone Rubber Ion-Selective Electrodes for Ultratrace Measurement. Anal. Chem. 2010, 86, 9425–9432.
- 10 Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128.
- 11 Wang, S.; Zhong, L.; Gan, S.; Tang, Y.; Qiu, S.; Lyu, Y.; Ma, Y.; Niu, L. Defective vs high-quality graphene for solid-contact ion-selective electrodes: Effects of capacitance and hydrophobicity. Electrochem. Commun. 2021, 129, 107091.
- 12 An, Q.; Jiao, L.; Jia, F.; Ye, J.; Li, F.; Gan, S.; Zhang, Q.; Ivaska, A.; Niu, L. Robust single-piece all-solid-state potassium-selective electrode with monolayer-protected Au clusters. J. Electroanal. Chem. 2016, 781, 272–277.
- 13 Wang, S.; Zhong, L.; Gan, S.; Tang, Y.; Qiu, S.; Lyu, Y.; Ma, Y.; Niu, L. Defective vs high-quality graphene for solid contact ion-selective electrodes: Effects of capacitance and hydrophobicity. Electrochem. Commun. 2021, 129, 107091.
- 14 Tang, Y.; Gan, S.; Zhong, L.; Sun, Z.; Xu, L.; Liao, C.; Lin, K.; Cui, X.; He, D.; Ma, Y.; Wang, W.; Niu, L. Lattice proton intercalation to regulate WO3-based solid-contact wearable pH sensor for sweat analysis. Adv. Funct. Mater. 2022, 32, 2107653.
- 15 Michalska, A. All-Solid-State Ion Selective and All-Solid-State Reference Electrodes. Electroanalysis 2012, 24, 1253–1265.
- 16 Guo, J.; Amemiya, S. Voltammetric heparin-selective electrode based on thin liquid membrane with conducting polymer-modified solid support. Anal. Chem. 2006, 78, 6893–6902.
- 17 Kim, Y.; Amemiya, S. Stripping analysis of nanomolar perchlorate in drinking water with a voltammetric ion-selective electrode based on thin-layer liquid membrane. Anal. Chem. 2008, 80, 6056–6065.
- 18 Hupa, E.; Vanamo, U.; Bobacka, J. Novel Ion-to-Electron Transduction Principle for Solid-Contact ISEs. Electroanalysis 2015, 27, 591–594.
- 19 Vanamo, U.; Hupa, E.; Yrjana, V.; Bobacka, J. New Signal Readout Principle for Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2016, 88, 4369–4374.
- 20 Han, T.; Mattinen, U.; Bobacka, J. Improving the Sensitivity of Solid-Contact Ion-Selective Electrodes by Using Coulometric Signal Transduction. ACS Sens. 2019, 4, 900–906.
- 21 Han, T.; Mattinen, U.; Mousavi, Z.; Bobacka, J. Coulometric response of solid-contact anion-sensitive electrodes. Electrochim. Acta 2021, 367, 137566.
- 22 Han, T.; Mousavi, Z.; Mattinen, U.; Bobacka, J. Coulometric response characteristics of solid contact ion-selective electrodes for divalent cations. J. Solid State Electr. 2020, 24, 2975–2983.
- 23 Han, T.; Vanamo, U.; Bobacka, J. Influence of Electrode Geometry on the Response of Solid-Contact Ion-Selective Electrodes when Utilizing a New Coulometric Signal Readout Method. ChemElectroChem 2016, 33, 2071–2077.
- 24 Lyu, Y.; Han, T.; Zhong, L.; Tang, Y.; Xu, L.; Ma, Y.; Bao, Y.; Gan, S.; Bobacka, J.; Niu, L. Coulometric ion sensing with Li+-selective LiMn2O4 electrodes. Electrochem. Commun. 2022, 139, 107302.
- 25 Jarolimova, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive Model for Coulometric Readout of Ion-Selective Electrodes. Anal. Chem. 2018, 90, 8700–8707.
- 26 Kondratyeva, Y. O.; Tolstopjatova, E. G.; Kirsanov, D. O.; Mikhelson, K. N. Chronoamperometric and coulometric analysis with ionophore- based ion-selective electrodes: A modified theory and the potassium ion assay in serum samples. Sens. Actuators B: Chem. 2020, 310, 127894.
- 27 Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ion-selective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142.
- 28 Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive Seawater pH Measurement by Capacitive Readout of Potentiometric Sensors. ACS Sens. 2020, 5, 650–654.
- 29 Kraikaew, P.; Sailapu, S. K.; Bakker, E. Rapid Constant Potential Capacitive Measurements with Solid-Contact Ion-Selective Electrodes Coupled to Electronic Capacitor. Anal. Chem. 2020, 92, 14174–14180.
- 30 Bondar, A.; Mikhelson, K. Constant potential coulometric measurements with Ca2+-selective electrode: analysis using calibration plot vs. Analysis using the charge curve fitting. Sensors 2022, 22, 1145.
- 31 Bondar, A.; Keresten, V.; Mikhelson, K. Registration of small (below 1%) changes of calcium ion concentration in aqueous solutions and in serum by the constant potential coulometric method. Sens. Actuators B: Chem. 2022, 354, 131231
- 32 Crespo, G. A.; Mistlberger, G.; Bakker, E. Electrogenerated Chemiluminescence for Potentiometric Sensors. J. Am. Chem. Soc. 2012, 134, 205–207.
- 33 Gao, W.; Jeanneret, S.; Yuan, D.; Cherubini, T.; Wang, L.; Xie, X.; Bakker, E. Electrogenerated Chemiluminescence for Chronopotentiometric Sensors. Anal. Chem. 2019, 91, 4889–4895.
- 34Ding, J.; Lv, E.; Zhu, L.; Qin, W. Optical Ion Sensing Platform Based on Potential-Modulated Release of Enzyme. Anal. Chem. 2017, 89, 3235–3239.
- 35 Hun X.; Xiong, X.; Ding, J.; Qin, W. Photoelectric current as a highly sensitive readout for potentiometric sensors. Chem. Commun. 2020, 56, 3879
- 36 Arsov, L.; Plieth, W.; Koßmehl, G. Electrochemical and Raman spectroscopic study of polyaniline; influence of the potential on the degradation of polyaniline. J. Solid State Electr. 1998, 2, 355–361.
Citing Literature
15 January 2023
Pages 207-213




