Kinetics of the Rhodium-Catalyzed Hydroaminomethylation of 1-Decene in a Thermomorphic Solvent System
Abstract
The homogenously rhodium-catalyzed tandem reaction of the hydroaminomethylation of 1-decene is investigated in a thermomorphic solvent system consisting of methanol and n-dodecane with the ligand sulfoxantphos. The influence of temperature, pressure and catalyst concentration is studied experimentally as the basis for kinetic modeling and parameter estimation. A kinetic model for the hydroaminomethylation is developed by connecting and reparametrizing a mechanistic approach for the hydroformylation with a reductive amination model. Simulations and experimental data are in good agreement indicating the transferability of mechanistic kinetic models.
1 Introduction
Amines represent important products and intermediates in the value chain of pharmaceuticals, agrochemicals, polymers, detergents and dyes. The hydroaminomethylation (HAM) is a one-pot tandem amine synthesis. Therefore, a purification of the intermediates is not necessary. In addition, the reaction has a high atom-economy since water is the only formed co-product. Industrially applied amine synthesis are often multistep routes with toxic by-products 1, 2. Transition metals like rhodium or ruthenium enable a highly active homogeneous catalysis 3. In combination with bidentate phosphine ligands high selectivities of higher aliphatic amines from alkenes can be achieved 4.
The HAM couples effectively the hydroformylation (Hyfo) and the reductive amination (RA). The main reaction sequence starts with the aldehyde formation from the olefin reacting with syngas (CO/H2) at the catalyst (Hyfo). An equilibrium-limited condensation reaction of the aldehyde with the co-substrate amine to form the enamine follows. Afterwards, this intermediate is hydrogenated at the catalyst to the target amine (Fig. 1a). The equilibrium between enamine and imine can be avoided if a secondary amine is used as co-substrate.
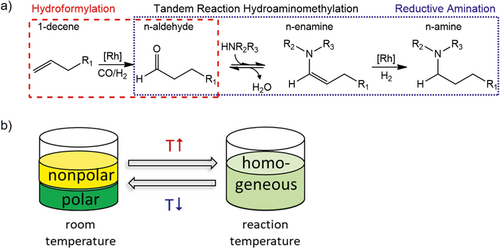
To attain an economic production the expensive rhodium catalyst has to be recycled carefully. One possibility is the application of thermomorphic solvent systems (TMS). They consist of at least one polar and one nonpolar solvent with a temperature-dependent miscibility gap. The idea is that the reaction takes place in a homogeneous mixture without liquid-liquid mass transport hindrance at reaction temperature. Subsequently, the product mixture is cooled down to separate it into two phases. Then the catalyst can simply be recycled with the polar phase 5-7. This principle is shown in Fig. 1b.
The key in design and optimization of chemical processes are reaction kinetics. Therefore, the Rh-catalyzed HAM of the model-substrate 1-decene with the ligand sulfoxantphos is experimentally and model-based investigated. The TMS consists of methanol and n-dodecane. In this contribution the influences of temperature, pressure and catalyst concentration on the reaction kinetics are studied.
2 Experimental
The experiments were carried out in a high-pressure, stainless-steel tank reactor (75 mL) with a magnetic glass stirrer, a gas feed system and a process controller for pressure and temperature. A solvent mixture of methanol/n-dodecane = 50:50 wt % was used. First, precatalyst Rh(acac)(cod), ligand sulfoxantphos (Rh/L = 1:3.5 mol %), methanol, n-dodecane and co-substrate diethylamine (DEA/1-decene = 8:7 mol %) were weighed into the reactor. To remove any oxygen the gas phase of the reactor was purged with nitrogen. For catalyst preforming 10 bar of syngas (CO/H2 = 1:2) were applied to the reactor before heating up to reaction temperature. To start the experiment the substrate 1-decene was injected with the reaction pressure. Keeping total pressure and temperature constant semi-batch operation conditions were realized. Samples were taken from the liquid phase via a capillary and analyzed with a gas chromatograph using a flame ionization detector. After 180 min, the experiment was finished. To guarantee reproducibility the kinetic experiments were repeated. In any case, the variation coefficient of the product amine concentration after 180 min was < 2 %. The experimental procedure and the analytics are described in detail in the Supporting Information (SI) and in other contributions 8-10.
2.1 Reaction Network
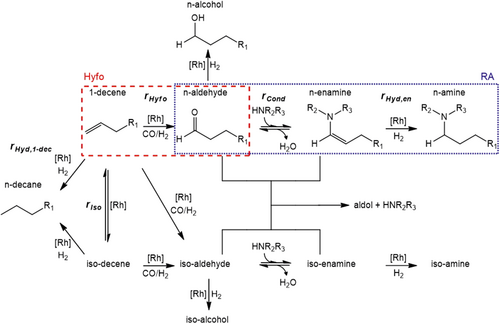
The reaction network of the HAM as a tandem reaction consists of the reaction sub-networks of the Hyfo 9 and the RA 11. These reactions and all of their side reactions are depicted in Fig. 2. In addition to the already described HAM main path, the isomerization of 1-decene to decene isomers with an internal double bond occurs. The iso-decenes can be hydroformylated to iso-aldehydes. In general, the substrate 1-decene can be converted to non-linear iso-aldehydes through an iso-Hyfo. The iso-aldehydes react with the co-substrate DEA in a condensation reaction to iso-enamines, which can finally be hydrogenated to iso-amines. As simplification, all the isomers were lumped together into the pseudo-components iso-decene, iso-aldehyde, iso-enamine and iso-amine 6, 12, 13. In our work the iso-enamines could not be detected in the gas chromatograph analysis but are assumed to be formed according to the linear main reaction route. The decenes and aldehydes can be hydrogenated at the catalyst to form n-decane and alcohols, respectively. Aldehyde and enamine form aldols. In all our experiments no significant formation of iso-aldehydes from 1-decene could be identified. Whenever 1-decene was almost fully consumed and iso-decenes were still present in the reaction mixture no significant n-decane formation could be observed. This leads to the conclusion that n-decane is formed mainly or maybe even exclusively from the hydrogenation of 1-decene. The resulting HAM reaction network coincides with other suggestions 6, 14.
2.2 Experimental Results
In preliminary experiments the influence of the catalyst amount on the HAM reaction kinetics was studied (see SI). Afterwards, the influence of pressure was investigated. The results depicted in Fig. 3a reveal that an increase in pressure above 30 bar does not affect the reaction kinetics significantly. Considering CO and H2 as reactants for Hyfo and enamine hydrogenation, higher reaction rates are expected when their partial pressures and corresponding dissolved concentrations are higher. This leads to the conclusion that a counteracting effect decelerates the reaction kinetics. Inhibition by CO is responsible for that. The Hyfo reaction mechanism is assumed as postulated by Jörke et al. and Kiedorf et al., in which the active monocarbonyl catalyst species is in equilibrium with an inactive dicarbonyl species 9, 12. A higher partial pressure of CO leads to a lower amount of the active species. Thus, a low partial pressure of CO increases the reaction rates of all Rh-catalyzed reactions causing a gas transport limitation for low pressures as shown in Fig. 3 for 20 bar and 15 bar. Due to the low pressure, the solubilized amount of CO is low, resulting in high reaction rates. Thus, CO is consumed faster by the Hyfo reaction than it is transferred from the gas phase into the liquid phase. As a result, the CO concentration decreases even more. The effect is self-reinforcing. Consequently, a low concentration of CO in combination with a high concentration of active catalyst promotes the other catalyzed reactions, especially the isomerization of 1-decene to iso-decene (Fig. 3b). Despite lower partial pressures of H2, the hydrogenation of 1-decene to n-decane increases for lower total pressures because of the high amount of active catalyst (Fig. 3c). These effects lead to a faster consumption of 1-decene at lower pressures. At higher conversions of 1-decene, the Hyfo rate decreases due to the missing substrate, entailing an increasement of the CO concentration. This leads to a further deceleration of the reaction rate until the equilibrium solubility of CO is reached.
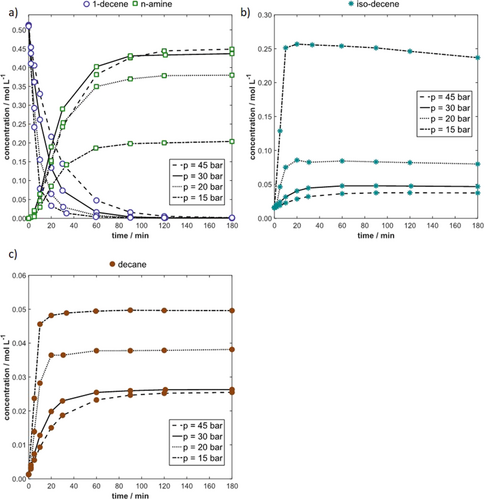
The Hyfo of iso-decene is negligibly slow compared to the Hyfo of 1-decene. A significant reverse isomerization from iso-decene to 1-decene could not be observed. A promoted isomerization due to a gas transport limitation decreases the selectivity of the target product n-amine drastically and should be avoided. Therefore, the results of experiments with a total pressure ≥ 30 bar were used exclusively for the kinetic evaluation to avoid the gas transport limitation regime. The conversion of 1-decene and yield of n-amine for each experiment are summarized in the SI (Tab. S2) including the variation of temperature.
3 Kinetic Description and Modeling
To decrease the level of complexity, the whole reaction network (Fig. 2) was reduced based on the performed preliminary experiments. The original network contains 16 components. As a first approach the alcohols, aldols and all iso-components and their formation rates were neglected due to their low occurrence in all experiments. Among these species iso-amine had the highest yield, being always smaller than 3.1 %. Due to the low concentrations of the reactive intermediates aldehyde and enamine, the formation rates of the side products aldols and alcohols are slow, which is a major advantage of the tandem reaction HAM. As mentioned in Sect. 2.1 the hydrogenation of iso-decenes to n-decane could not be observed in the experiments and is thus neglected. This leads to the final reduced network applied for modeling, depicted in Fig. 4. It consists of 10 components and 5 reactions, two of them being reversible.
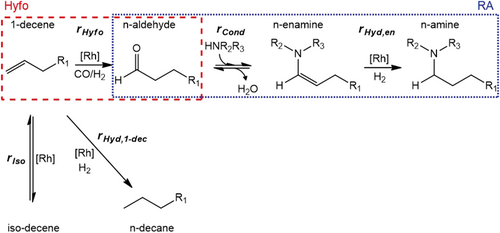
The model description is summarized in detail in the SI. For the Hyfo sub-network mechanistic rate approaches, considering the distribution of the catalytic material within the catalytic cycle on different species, were already derived for a similar system (Rh-Biphephos-catalyzed Hyfo of 1-decene in DMF/n-dodecane) 9, 13, 15. These are applied here for the kinetic description of the Hyfo and its side reactions. The rate equations for the RA sub-network are adopted with an equilibrium-limited approach for the condensation and a power law approach for the hydrogenation of the enamine 8. For this first model the mechanistic and equilibrium constants in Eqs. (S8)–(S12) were used as already published 8, 9, 13 to reduce the amount of parameters to a reasonable number of 10. Although, another TMS (DMF/n-dodecane) and bidentate ligand (Biphephos) were used by Jörke et al., it is assumed that there is no significant change in the equilibrium constants due to the similarity of the systems. Consequently, only the collision factors and the activation energies of the five reactions shown in Fig. 4 were estimated using MATLAB®. Further information about the model and parameter estimation can be found in the SI.
Selected results of the simulations of the temperature influence are depicted in Fig. 5. The simulated results are in good agreement with the experiments for the substrate 1-decene, the main product n-amine (Fig. 5a) and the side products n-decane and iso-decene (Fig. 5b) as well.
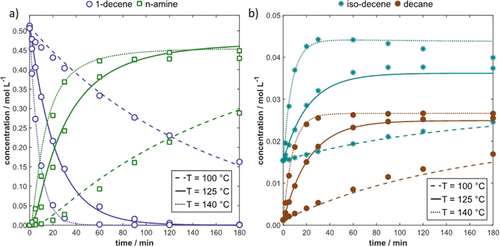
4 Discussion and Outlook
Based on experimental investigations of the homogeneously rhodium-catalyzed HAM of 1-decene in a TMS, considering the influences of pressure, temperature and catalyst concentration, a first kinetic model was developed. In a first step, the reaction network was reduced to consider just the relevant components and reactions. An existing mechanistic kinetic model for the Hyfo was transferred to the tandem reaction HAM by reparametrizing the collision factor and activation energy and extended with a RA model for the overall HAM description. The simulations are in good agreement with the experimental semi-batch runs with respect to the substrate and the final products. This reveals the transferability and advantage of the developed mechanistic models for the Hyfo sub-network. A further improvement of the kinetic description, especially with respect to the intermediates, should be achievable by taking a temperature-dependent equilibrium constant for the condensation reaction and a mechanistic reaction rate expression for the RA into account.
Supporting Information
Supporting Information for this article can be found under DOI: https://doi.org/10.1002/cite.202100180.
Acknowledgements
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – TRR 63 “Integrierte chemische Prozesse in flüssigen Mehrphasensystemen” (Teilprojekt A3) – 56091768. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – TRR 63 “Integrated Chemical Processes in Liquid Multiphase Systems” (subproject A3) – 56091768. We are grateful for the supply of the catalyst precursor from Umicore.
Symbols used
-
- p [bar]
-
pressure
-
- r [mol L−1min−1]
-
reaction rate
-
- T [°C, K]
-
temperature
-
- x [mol %]
-
molar fraction
Sub- and Superscripts
-
- 1-dec
-
1-decene
-
- cat
-
catalyst
-
- Cond
-
condensation
-
- en
-
enamine
-
- Hyd,1-dec
-
hydrogenation of 1-decene
-
- Hyd,en
-
hydrogenation of n-enamine
-
- Iso
-
isomerization
-
- reaction
-
after substrate is added
Abbreviations
-
- acac
-
acetylacetonato
-
- cod
-
1,5-cyclooctadiene
-
- DEA
-
diethylamine
-
- DMF
-
dimethylformamide
-
- HAM
-
hydroaminomethylation
-
- Hyfo
-
hydroformylation
-
- L
-
ligand
-
- RA
-
reductive amination
-
- TMS
-
thermomorphic multiphase solvent system




