Direct cellular reprogramming and transdifferentiation of fibroblasts on wound healing—Fantasy or reality?
Edited by Yi Cui
Abstract
Induced pluripotent stem cell (iPSC) technology is one of the de novo approaches in regeneration medicine and has led to new research applications for wound healing in recent years. Fibroblasts have attracted wide attention as the first cell line used for differentiation into iPSCs. Researchers have found that fibroblasts can be induced into different types of cells in variable mediums or microenvironments. This indicates the potential “stem” characteristics of fibroblasts in terms of direct cellular reprogramming compared with the iPSC detour. In this review, we described the morphology and biological function of fibroblasts. The stem cell characteristics and activities of fibroblasts, including transdifferentiation into myofibroblasts, osteogenic cells, chondrogenic cells, neurons, and vascular tissue, are discussed. The biological values of fibroblasts are then briefly reviewed. Finally, we discussed the potential applications of fibroblasts in clinical practice.
Highlights
-
We described the morphology and biological functions of fibroblasts and discussed the stem cell characteristics and activities of fibroblasts, including transdifferentiation into myofibroblasts, osteogenic cells, chondrogenic cells, neurons, and vascular tissue.
-
The biological value of fibroblasts is also briefly reviewed.
-
Finally, we provided an overview of the potential application of fibroblasts in clinical practice.
1 INTRODUCTION
Stem cell techniques have become a recent focus in biomedicine, particularly methods using induced pluripotent stem cells (iPSCs). Among the de novo approaches in regeneration medicine, iPSC is one such technology and has led to new research applications in wound healing. The first iPSCs were reprogrammed from mouse tail fibroblasts in 2006.1 Fibroblasts have been the most used primary somatic cells for the generation of iPSCs. Fibroblasts have thus been the subject of attention in regeneration medicine because they were the first cell line used for differentiation into iPSC. Subsequent studies showed that fibroblasts can be differentiated directly into many types of cells, including myofibroblasts, osteogenic cells, chondrocytes, neurons, vascular endothelial cells, cardiac cells,2 hepatocytes,3 brown adipocytes,4, 5 and pancreatic β cells6 (Figure 1). These findings led to the development of an approach using direct cellular reprogramming rather than iPSC-based reprogramming, which is a time-consuming and costly approach. Direct reprogramming, thus, is a promising alternative to rapidly prepare different cell types by bypassing the pluripotent state.
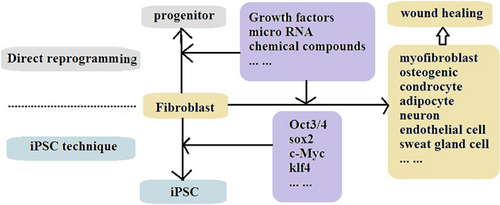
As new approaches are established in basic biomedical research, their applications in the medical field and patient treatment are explored. The direct cellular reprogramming technique has been widely used in wound healing and other tissue regeneration studies. Although the publications on the application of fibroblasts in direct cellular reprogramming for wound healing have been limited, the development of this technique for wound healing management is ongoing. In this review, the characteristics and biological functions of fibroblasts will be described. The research evidence and ongoing studies of fibroblasts in direct cellular reprogramming, including transdifferentiation into myofibroblasts, osteogenic cells, neurons, and vascular cells, will be reviewed. The biological value of fibroblasts as potential stem cells is then presented. Finally, we discuss the critical roles of fibroblasts in regeneration medicine and wound healing.
2 GENERAL INTRODUCTION OF FIBROBLASTS
There are abundant sources of fibroblasts in both animals and humans. Fibroblasts can be derived from many different organs and tissues, including skin, cardiac, lung, liver, and kidney.7 Dermal fibroblasts can be easily acquired and are used widely. Several types of mesenchymal cells, such as adipocytes, can be differentiated into fibroblasts. We also used mouse embryonic fibroblasts in our previous study.8 The processes among the differentiation of fibroblasts may vary. Fibroblast diversity was further demonstrated by expression data, which showed that gene expression varies depending on the site from which cells are derived. For example, HOX gene expression pattern in fibroblasts appears to reflect the body site from which the cells are derived. Even at the same local site, dermal fibroblasts from different layers of human skin showed differences in collagen I and collagen III messenger RNA (mRNA) expression; fibroblasts from deeper layers showed reduced collagen expression compared with cells from more superficial layers.9
Fibroblast phenotypes show large variability. In general, the morphology of fibroblasts is not regular and shows a deer horn shape or spindle shape; the cells are also negative for α-smooth muscle actin (α-SMA),10 which is an early differentiation marker of smooth muscle cells. The phenotype of a fibroblast cell line cultured in vitro under an inverted phase contrast microscope is shown in Figure 2.

Fibroblasts are the major cells responsible for the synthesis of collagen in the skin and most other tissues. Fibroblasts contribute to normal tissue homeostasis by regulating the composition of the extracellular matrix (ECM). Some mediators, including growth factors, lymphokines, enzymes, other chemokines, and even bionanomaterials, can influence fibroblast synthesis of collagen and matrix constituents in wound healing and tissue fibrosis. Among these biological and chemical substances, transforming growth factor β (TGF-β) was shown to play a key role in activating fibroblast synthesis of collagen in wound repair, but γ-interferon inhibited the same synthesis process.11 Fibroblasts are in a resting state in normal skin but are activated during the wound repair process. Upon injury, fibroblasts proliferate and migrate into the wound site, synthesize new connective tissue, and contract it to facilitate wound closure. The function of fibroblasts is primarily collagen deposition in the dermal wound area, along with the production of type III collagen and fibronectin within the first 3 days after tissue injury. Fibroblasts also secrete cytokines that attract keratinocytes to the injury site for re-epithelialization. The proliferative stage terminates with the breakdown of ECM and leads to reduced hyaluronic acid and increased chondroitin sulfate, which then gradually triggers the fibroblasts to stop migrating and proliferating.12
3 RESEARCH OF THE STEM CELL CHARACTERISTICS OF FIBROBLASTS IN DIRECT CELLULAR REPROGRAMMING
Research from over 10 years ago showed that fibroblasts can be induced into other types of cells in the laboratory. While it will be a long road for the application from bench to bedside, we envision the capability for the potential differentiation of fibroblasts as a mimic stem cell. Many studies have described the ability to directly convert fibroblasts into functional myocytes, osteogenic cells, chondrocytes, cardiac muscle cells, neurons, hematopoietic cells, and megakaryocytes.13
3.1 Differentiation of fibroblasts into myofibroblasts and promoted skin wound healing
Wound healing is a complex and multistep process that requires the integration of activities of variety of tissues and cells. The phase of re-epithelialization is a crucial process during early wound healing; this occurs through the migration and proliferation of keratinocytes in the epidermal layer from the wound edge and by the differentiation of some stem cells and fibroblasts in the dermal layer. Rapid re-epithelialization after skin injury can provide an optimum microenvironment for healing, including the scaffold function and various cytokines, including multiple growth factors that are indispensable for the healing process. Wound contraction is another important process in the early wound-healing stage. It minimizes the open area by pulling the neighboring tissue toward the wound center. Myofibroblasts are found in the early phase of granulation tissue formation; they become abundant during the proliferation phase and disappear gradually through apoptosis. Through the contraction of their actin cytoskeleton, myofibroblasts at the wound site reduce the initial size of the three-dimensional (3D) collagen matrix, thereby contributing to tissue repair. In this process, myofibroblasts generate the contractile force by which the wound area contracts.
The first case of direct reprogramming was demonstrated when fibroblasts were converted into myofibroblasts by transfection of myogenic differentiation I (Myo D1) cDNA. Some of the fibroblasts infiltrating the wound differentiate and become myofibroblasts that have bundles of α-SMA and can contract, thus contributing to the closure of the wound. Other results demonstrated that the chicken chemotactic and angiogenic factor-stimulated fibroblasts differentiate into myofibroblasts and produce high levels of α-SMA.14 The study also found that in human injury joint capsule fibroblasts from orthopedic surgery that were cultured in vitro, the typical spindle-like shape increasingly changed toward the phenotype of stellate cells, and the cells were strongly positive for α-SMA.15
The transformation of fibroblasts into contractile myofibroblasts is a key element in the early phase of wound repair. Several studies have investigated the mechanisms by which fibroblasts become myofibroblasts, and the two most important stimuli are electrical stimulation and TGF-β.16, 17 Exposure to electrical stimulation promotes skin fibroblast growth and migration, increases growth factor secretion, and promotes fibroblast transdifferentiation into myofibroblasts, therefore leading to wound healing.18 TGF-β1-induced telomere dysfunction causes fibroblasts to transdifferentiate into α-SMA-expressing myofibroblasts. The myofibroblasts acquired the ability to contract collagen lattices and displayed a gene expression signature characteristic of function. The formation of dysfunctional telomeres and downstream p53 signaling was necessary for myofibroblast transdifferentiation. Inducing telomere dysfunction using shRNA against TRF2 also caused cells to develop features characteristic of myofibroblasts, even in the absence of exogenous TGF-β1.19
During development, ectodermal cells depend on instructive signals from the underlying mesenchyme to first commit to the epithelial lineage and subsequently form the fully differentiated epidermal with skin appendages. Therefore, epithelial–mesenchymal interactions play a crucial role in skin development and cutaneous tissue repair. In one study, fibroblasts were cocultured with keratinocytes in a complex tissue culture model to examine how cells responded to epithelial stimuli during the wound healing process. The results showed that numerous ECM and smooth muscle cell-associated gene transcripts were upregulated in fibroblasts, suggesting the differentiation of fibroblasts into myofibroblasts. Differentiation required endogenous TGF-β and was inhibited by interleukin 1 and wingless-type MMTV integration site family, member 4.20, 21 This may be one of the pathophysiological mechanisms of anti-inflammation in clinical wound management.
In our previous mouse model study, we found that silver nanoparticles (AgNPs) could mediate and promote keratinocyte proliferation at the wound site. In contrast, AgNPs suppressed fibroblast proliferation, with a subsequent drive toward differentiation into myofibroblasts. These results were the first to show that metal nanoparticles can elicit differential effects toward distinct cell types.8 This is an important issue because the ability to manipulate stem-like cells would open a new route in terms of tissue regeneration. These novel techniques have laid the road from basic research to clinical application for wound management.
3.2 Fibroblasts differentiate into osteogenic cells and have potential value for bone fracture
The repair of bone defects by grafting can be performed using natural or biosynthetic materials. Strategies for bone tissue regeneration to achieve restoration require productive sources of osteoblasts, which are responsible for bone formation. However, native osteoblasts are typically difficult to isolate and expand in vitro, leading to a less-than-ideal number of cells for bone regeneration technique. Therefore, an alternative source of cells that can be used as osteoblasts is desired. The ability of fibroblasts to undergo differentiation into osteoblasts has been demonstrated by some researchers.22-26 Rheumatoid arthritis synovial fibroblasts travel from cartilage destruction sites to new locations where they reinitiate the destructive processes at distant articular cartilage surfaces and affect the function of joints.27 One study explored the mRNA levels of tumor necrosis factor-α, vessel endothelial growth factor, and matrix metalloproteinase-3 (MMP-3) in synovial tissues in ankylosing spondylitis, another rheumatism disease, to analyze the functions of these proteins in the differentiation of the synovial tissue fibroblasts into osteoblasts and osteoclasts. The results indicated that all three factors directly participate in the differentiation of fibroblasts into osteoblasts.28 Therefore, the pathological mechanism of one disease could become the new paradigm of the basic physiological principle for the treatment of another disease.
Cells of the fibroblast lineage, specifically dermal fibroblasts, can express osteoblast activity markers. Some cytokines such as bone morphogenetic protein-2, TGF-β1, basic fibroblast growth factor, vascular endothelial growth factor, and 1,25-(OH)2D3 play a role in the formation of bone tissue, including both the fibroblast recruitment and its differentiation.29, 30 Bone tissue engineering is also dependent on the use of signaling molecules that influence cell growth. Several factors, such as dexamethasone, ascorbic acid, and β-glycerophosphate, have been found to stimulate osteoblast differentiation and bone maturation. In some cases, fibroblasts, osteoblasts, and chondrocytes concomitantly express the same cytokine receptors, such as the integrin receptor, which can modulate critical cellular processes, including adhesion, migration, survival, ECM organization, and differentiation. These processes are physiologically related to growth, development, and tissue homeostasis and may even be significant in pathological conditions such as organ fibrosis.31 Further studies are required to clarify the underlying molecular mechanisms.
Previous studies have shown that nanoparticles, such as AgNPs, can promote direct reprogramming. With its antibacterial function, AgNPs may become the ideal promoter for fibroblast reprogramming and wound management. We showed that AgNPs promoted mesenchymal stem cells to differentiate into osteogenic cells and accelerated the fracture-healing process in a mouse model.32 Both mesenchymal stem cells and fibroblasts are developed from the same mesoderm, and we further observed that AgNPs could promote fibroblast transdifferentiation into osteogenic cells in vitro (Figure 3). Although the mechanism is not well known, this result provides evidence for the use of AgNPs to promote fibroblast transdifferentiation and treat bone fractures. Together, the results of these studies suggest that fibroblasts possess osteogenic transdifferentiation potential capability and might be an alternate source of cells for bone repair.
Joint cartilage disease is another commonly encountered issue in clinical practice. The articular cartilage is vascular tissue composed of chondrocytes embedded in the ECM to form the basis of smooth articulation of the joint. The direct conversion of fibroblasts into chondrocytes provides a slight advantage compared with the iPSC approach. In contrast to iPSC technique-mediated generation, the direct reprogramming of fibroblasts into chondrocytes has not been extensively explored. However, this process actually takes place in 3D cell culture to prevent dedifferentiation. Fibroblast differentiation into chondrocytes could be promoted by some transform factors and also by c-Myc, Klf4, and Sox9 expression.33 Another challenge and clinical problem is repairing cartilage injury with hyaline cartilage. Tsumaki and his group induced cartilaginous hyaline directly from adult mouse dermal fibroblast culture.34, 35 They then induced chondrogenic cells from human dermal fibroblast culture.36 These approaches provide a means for the clinical application of cartilage defects.
Previous studies have shown that primary fibroblast-like cell populations can differentiate into at least one mesenchymal lineage to generate osteoblasts, chondrocytes, or adipocytes. Furthermore, the ability of different cell populations to differentiate into particular lineages appeared to depend on the source tissue. For example, fibroblasts derived from the lung and amniotic membrane could transdifferentiate efficiently into osteoblasts and chondrocytes, while those derived from the umbilical cord could transdifferentiate into chondrocytes and adipocytes, but the degree of differentiation appeared to be lower than that from other tissues. Only fibroblasts from adult skin could transdifferentiate into osteoblasts, chondrocytes, and adipocytes. Therefore, the skin tissue may serve as an additional fibroblast source of “pluripotent” expanding cells in adult individuals for therapies on the basis of regenerative medicine.37
3.3 Fibroblasts can be differentiated into neurons and vascular cells for tissue regeneration and promote wound healing
The induction of some target cells, such as neurons, cardiomyocytes, islet β-cells, and liver cells, is difficult because of their complex electrophysiological and endocrine functions. However, the direct programming from fibroblasts could represent a new strategy for this induction.
Neural reprogramming is one of the most advanced research fields in direct reprogramming. Researchers have shown that both human and animal dermal fibroblasts can be converted into functional excitatory neurons using a combination of neuronal lineage-specific transcription factors.38-40 In some cases, it is not possible to achieve similar transdifferentiation efficiencies in human fibroblasts using identical combinations of transcription factors, microRNA, and small molecules that have been found to convert murine cells into neurons. Brn2, Ascl1, and Mytl1 were sufficient to reprogram murine cells into functional neurons but failed to induce similar reprogramming in human fetal fibroblasts.41 Although the fibroblasts were converted to motor neurons in vitro, the conversion efficiency was still low. Human fibroblasts have also been proven to be more difficult to reprogram than murine fibroblasts. Human fibroblasts require more transcription factors, and the process is slower and less efficient compared with that for murine cells. Therefore, some new methods have been explored to reprogram human fibroblasts directly to neurons. Biophysics has become a research hotspot in cell reprogramming. Physical factors such as radiofrequency microcurrent, nanotopography, and electromagnetic force have been used for cell reprogramming. Because of the unique advantages of physical factors in the process of reprogramming human fibroblasts into neurons, such as the safe and minimally invasive nature of these techniques, this method has a promising application prospect.42 These results suggested that direct reprogramming was possible even between different lineages or germ layers, for example, from mesodermal cells into ectodermal cells. Several groups subsequently reported on neural direct reprogramming in mice embryonic fibroblasts, human postnatal fibroblasts, and fibroblasts from patients with Parkinson's disease. The patient-specific induced neuronal cells obtained from direct reprogramming were similar to the normal human neurons in terms of morphology, surface antigens, gene expression, electrophysiological signals,43 aging hallmarks, and associated features.44
The direct reprogramming of adult fibroblasts into neuronal-like cells was also achieved in vitro with forced expression of a combination of transcription factors, microRNA,45 and small molecules.46 Ring and other researchers47 have reported on the generation of multipotent neural stem cells from mouse and human fibroblasts with overexpression of a single factor, SOX2. SOX2 is a key transcription factor expressed in pluripotent embryonic stem cells. It is expressed widely in early neuroectoderm and neural progenitor cells during neural system development. Hence, many studies have reported direct reprogramming of fibroblasts into neural stem cells or precursors by ectopic SOX2. SOX2 is described as a “master regulator.”43, 48 A previous study49 reported that induced oligodendrocyte progenitor cells were derived from adult mouse fibroblasts by October 4, and the data in the study showed that the cells contributed to functional recovery in a spinal cord injury model. Thus, the regeneration of neurons has promising implications for clinical practice.
In studies on vascular regeneration, some groups reported that adult mouse fibroblasts could be directly reprogrammed into functional differentiated endothelial cells using a cocktail of factors, including Foxo1, Er71, Klf2, Tal1, and Lmo2. The induced endothelial cells mimicked the function of mature endothelial cells and released nitric oxide upon stimulation with acetylcholine or vascular endothelial growth factor. Furthermore, the induced endothelial cells enhanced angiogenesis and limb perfusion in a hindlimb ischemia murine model.50 These results demonstrated that the induced tissue mimicked not only the morphology but also the vascular function. More recently, Lee and his group51 sought to directly reprogram human postnatal dermal fibroblasts into endothelial cells with vasculogenic and endothelial transcription factors and then determined the vascularizing and therapeutic potential. The results showed that ER71/ETV2 alone could directly reprogram human postnatal cells into functional, mature endothelial cells after an intervening transgene-free period. These endothelial cells may be a valuable resource for cell therapy and wound regeneration.
Direct reprogramming cocktail factors, including transcription factors, microRNAs, small molecules, and combinations of these molecules, play a major role in the vascular reprogramming process. Optimizing the stoichiometry could increase the efficiency of reprogramming and improve the regeneration tissue function in vivo. In addition to the above-mentioned factors, unidentified extrinsic factors such as topographic cues, mechanical forces, growth factors, cytokines, and some paracrine signals also play critical roles in the reprogramming process. To more accurately mimic the in vivo environment, fibrin hydrogel 3D culture increased both MMP and gene expression in a microRNA reprogramming cocktail.52 Smooth muscle cells are key components of tissue-engineered vessels. Karamariti's group53 obtained smooth muscle cells by direct reprogramming of human neonatal lung fibroblasts. The researchers developed a protocol to generate smooth muscle cells through a signaling pathway, and these cells may be useful for generating tissue-engineered vessels. Concurrently with fibroblast and keratinocyte migration, angiogenesis can occur and lead to the formation of new blood vessels, which is crucial for wound healing.12 Therefore, the regeneration of blood vascular cells and neurons could improve wound healing because of the supportive role of the tissue.
4 THE PRIORITY OF FIBROBLASTS PLAYING THE ROLE AS THE “STEM” CELL
The fibroblast can be obtained from the dermal layer and other various tissues and organs with noninvasive procedures. Fibroblasts can be easily biopsied from patients, and differentiation can be stimulated under sterile conditions. For example, dermal fibroblast samples can be easily harvested from small fragments of the patient's own skin without any decrease in collagen biosynthesis or growth level. These fibroblasts are thus potentially significant for clinical application. Additionally, as the potential alternative source of cells, fibroblasts are readily available, nonimmunogenic, avoiding host-versus-graft rejection, and are easily expandable both in vitro and in vivo. Therefore, fibroblasts can avoid possible immunological rejection and ethical problems. Compared with technologies that use embryonic stem cells, mesenchymal stem cells, hematopoietic stem cells, and iPSCs, which may have ethical concerns, risk of tumor occurrence, time-consuming techniques, and cost, direct cellular reprogramming of fibroblasts has emerged as a promising approach with a fast conversion rate and lower teratoma risk. The direct injection of defined factors can avoid the need for cell transplantation, in which long-term cell survival might be challenging in some organs.
Attempts have been made to reprogram fibroblasts for a wide array of functional cell types. Fibroblast-derived cell lines are widely used in biomedical research. The direct lineage reprogramming technique has been used in disease models, drug screening, personalized medicine, regeneration, and transplantation therapy. In one study, researchers performed a comparison of the capacity of fibroblasts and adipose-derived stem cells to proliferate and survive for tendon regeneration. The results showed that fibroblasts survived longer than adipose-derived stem cells in vivo. Hence, fibroblasts could improve chronic wound healing and are particularly feasible for use in tendon injury.54
5 THE POTENTIAL VALUE OF FIBROBLAST DIFFERENTIATION IN WOUND MANAGEMENT
Nonhealing wounds, such as diabetic wounds, diabetic foot ulcers, and press ulcers, are problems faced by both medical physicians and trauma surgeons in daily clinical settings. The traditional strategies that have been used to treat these types of wounds include vacuum-assisted wound closure, shock-wave therapy, electrical stimulation, and local warming. However, the results are neither satisfactory nor stable.
Fibroblasts surrounding the wound are important players in regulating dermal tissue homeostasis. When the skin is injured, fibroblasts migrate to the injured sites; they then differentiate into contractile myofibroblasts leading to wound closure and contributing to wound healing. However, the typical chronic nonhealing wound edges were not contractive since, in these sorts of wounds, fibroblasts usually failed to undertake differentiation in the dermis. The induction of fibroblast regulators fibroblast growth factor (FGF)-1 and FGF-2 in vivo occurred earlier in diabetic wounds than in normal wounds. The expression of both FGF-1 and FGF-2 mRNA returned to basal levels within 3 days after injury.55 Noizet and his team56 established a human ex vivo model of chronic wounds where fibroblasts undergo normal myofibroblast differentiation or take on a nondifferentiable pathological state. Another study presented the mechanism of promoting cutaneous wound contraction via fibroblast migration and differentiation to myofibroblasts.57 Deep and ischemic wounds usually impair dermal myofibroblasts because of the hypoxia state.58 Thus, inducing fibroblasts may support myofibroblast formation to improve ischemic and chronic wound healing.
Directed reprogramming and transdifferentiation are theoretically able to produce all desired cell types. Fibroblasts are ideal cell resources and play a key role in the repair of chronic nonhealing wounds. Sometimes, chronic skin ulcers are deep to the subcutaneous and muscle layer and even damage the bone and cartilage or joint. Therefore, if the fibroblasts around deep wounds have multipotential differentiation ability into both epidermal and dermal tissue, even subcutaneous and bone tissue, they would be the ideal “stem” cells suitable for nonhealing wound management. The functional maturity of reprogrammed fibroblasts may be improved by identifying and adding transcription factors and establishing an optimal cellular microenvironment. The in vivo microenvironment can provide an appropriate 3D setting for the functional maturation of the target cell types. Therefore, we could also join the fibroblasts and other cells for coculture by some cytokines, as well as combine the prior technique, for example, the 3D bio-printing59 or 3D tissue culture technique, to repair nonhealing wounds.
6 OUTLOOK OF THE CRITICAL ROLE OF FIBROBLASTS IN REGENERATION MEDICINE
The states of terminally differentiated cells were previously considered to be fixed and irreversible. Recent evidence has demonstrated that the balance of transcription factors can influence cell fate. Terminal differentiated cells show some degree of plasticity and are convertible to other cell lineages. Therefore, the direct reprogramming strategy is of high interest in basic biomedicine research and clinical applications to develop a new cell source for regenerative medicine and tissue engineering. The distinct direct reprogramming approach is a potential strategy to obtain new abundant cell sources for tissue regeneration and can meet the high demand for regeneration medicine strategies for wound healing.
Some work is still required to realize these goals. Continued research into key transcription factors, noncoding RNAs, small molecules, reprogramming mechanisms, delivery and targeting methods, and biomaterials will help advance direct reprogramming. Large animal models such as pigs, dogs, and sheep will be required in future studies, as they have a physiologic resemblance to humans with similar body size and structure of the skin.
Because of the demonstrated advantages of fibroblasts around wounds, strategies using these cells will be the direction of research in nonhealing wounds. Gene therapy approaches for nonhealing wounds are not well-researched. Some mechanisms and applications of fibroblasts as the direct reprogramming cell resources remain largely unknown. Detailed analyses of the properties of directly induced cells are necessary to be the subject of future research. The research results on nonhealing wound care are promising and may help to improve patient management in practice. Despite many challenges and hurdles in this emerging field, current findings indicate that fibroblasts represent an accessible and safe starting cell population for reprogramming techniques for regenerative medicine applications. The aim of translation medicine is to take the fibroblasts from bench to bed and improve the care of chronic wound patients as soon as possible.
Finally, a safe and efficient delivery system is required for the translation of direct reprogrammed cells to clinical use. In the field of regenerative medicine, the major goal is to convert resident tissue–specific cells into target cell types to replace the damaged tissue. Lineage direct reprogramming in situ regeneration and repairment in vivo are potential techniques. The future challenge is to tackle current drawbacks by pursuing further research on optimal reprogramming and culture microenvironment so as to prevent telomere dysfunction60 and tumorigenic, as well as fibrosis and hypertrophic activities. Additional techniques such as 3D bioprinting,59, 61 nano biomaterials,62, 63 electrospinning,64 and ionizing radiation63, 65 may promote direct reprogramming. The techniques for cellular direct reprogramming will improve these technologies and change the face of regenerative medicine and patient care.
AUTHOR CONTRIBUTIONS
Juan Du reviewed the literature and drafted the manuscript. Xuelai Liu and Carol Wing Yan Wong reviewed the literature and prepared the figures. Kenneth Kak Yuen Wong and Zhixin Yuan revised the manuscript. All the authors approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by Shenzhen Healthcare Research Project (No. 201505018), China; The Science and Technology Project of Jilin Province (No. 20210203079SF), China; The Creation and Innovation Project of Jilin Province (No. 2022C043-11), China; and The Health Technology Promotion Project of Jilin Province (No. 2022JC001), China.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
None.




