Harnessing natural killer cells to develop next-generation cellular immunotherapy
Siyao Liu, Kaycee Nguyen, and Dongyong Park contributed equally to this study.
Edited by Yi Cui
Abstract
Cellular immunotherapy harnesses the body's own immune system to fight cancer by using engineered T cells, macrophages, or natural killer (NK) cells. Compared to chimeric antigen receptor T (CAR-T) cells that are commonly used to treat hematological malignancies, CAR-NK cells have shown remarkable therapeutic effectiveness while exhibiting enhanced safety, reduced risk of graft-versus-host disease, fewer side effects, and amplified antitumor efficacy. Preclinical trials have unveiled the high potential of adoptive CAR-NK cell therapy to curtail or even eliminate both hematological malignancies and solid tumors in animal models. We brought forth herein the design principle of CAR-NK cells, highlighted the latest progress in the preclinical testing and clinical trials of CAR-NK cells, briefly delved into discussed major roadblocks in CAR-NK therapy, and discussed potential solutions to surmount these challenges. Given the accelerated progress in both basic and translational studies on immune cell engineering, CAR-NK cell therapy promises to become a serious contender and important addition to the next-generation cell-based immunotherapy.
Key points
-
CAR-NK cell therapy emerges as a promising immunotherapeutic option to curtail both hematological neoplasms and solid tumors by overcoming the resistant tumor microenvironment.
-
CAR-NK cell therapy exhibits a superior safety profile with effective cytotoxicity against tumor cells, making it a new addition to the fourth pillar of cancer therapy.
1 INTRODUCTION
For decades, cancer treatments have been mainly centered around surgery, radiation therapy, and chemotherapy. However, over the past 5 years, cellular immunotherapy has increasingly become the fourth pillar of cancer therapy that shows encouraging curative potential in certain types of hematological neoplasms, such as B-cell lymphoma, leukemia, and multiple myeloma.1 Cellular immunotherapy, as most prominently exemplified by the chimeric antigen receptor T (CAR-T) cell therapy, harnesses the body's immune system to specifically engage and eliminate cancer cells.1-4 The goal of cancer immunotherapies is to enhance the patient's immune response to detect and clear cancerous cells, especially cells with accumulated mutations that allow them to evade the host immune system. To achieve this, immune cells are engineered to express specific receptors, such as chimeric antigen receptors (CARs). A typical CAR consists of a signal peptide for directing the correct position and topology of the engineered receptor, an extracellular antigen-specific single-chain variable fragment (scFv), a hinge region to provide stability and flexibility to access the target antigen, a transmembrane domain, as well as a 4-1BB costimulatory domain and a CD3ζ signaling domain to mediate T-cell activation, expansion, and persistence. Usually, this antigen is specific to cancer tissues or overexpressed in tumor cells when compared to normal tissues. For instance, among the six FDA-approved CAR-T cell therapies, four products (Kymriah, Yescarta, Tecartus, and Breyanzi) target the CD19 antigen overexpressed in leukemia or lymphoma cells to treat acute lymphoblastic leukemia (ALL) and relapsed or refractory diffuse B-cell lymphoma (DLBCL), whereas the remaining two products (Abecma and Carvykti) target the B-cell major antigen (BCMA) to treat multiple myeloma.1, 2, 5-10
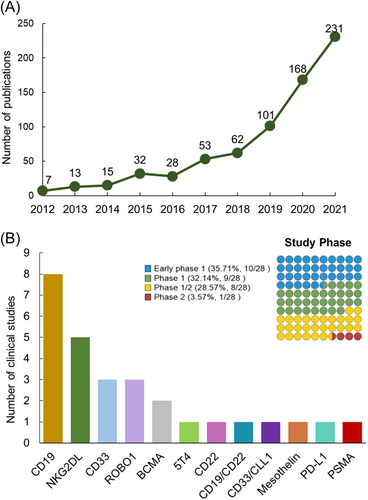
The introduction of a synthetic scFV into CAR enables engineered immune cells to recognize tumor cells in an MHC-unrestricted manner. Upon engagement with tumor antigen, CAR-T cells are activated to perform effector functions by activating several downstream signaling pathways, including the Fas/FasL axis, the perforin-granzyme axis, and cytokine release to sensitize the tumor microenvironment (TME). Recent CAR constructs consist of targeting domains and/or costimulatory domains that have been shown to improve cytotoxic function and induce rapid proliferation.1 CAR-T cells have shown remarkable efficacy in patients with relapsed or refractory hematological malignancies.1-4 However, their wide application is limited by several non-negligible side effects and/or inherent drawbacks, including graft-versus-host-disease (GvHD), cytokine release syndrome (CRS), potential “on-target off-tumor” cytotoxicity, and failure of efficient infiltration into solid tumors due to the immunosuppressive TME.11, 12 CAR-expressing NK (designated “CAR-NK”) cells, nevertheless, are capable of overcoming some of these roadblocks because of their advantages over CAR-T cells in killing malignant cells with fewer adverse side effects after transplantation.13, 14 Therefore, they can be exploited as an alternative therapeutic option.15, 16 Over the past 10 years, we have witnessed a steady increase of studies centered on CAR-NK cells (Figure 1 and Table 1).
| NCT number | Tumor type(s) | Antigen(s) | Phase(s) | Status |
|---|---|---|---|---|
| NCT03056339 | B-cell lymphoma; ALL; CLL | CD19 | Phase 1/2 | Recruiting |
| NCT03690310 | B-cell lymphoma | CD19 | Early Phase 1 | Unknown |
| NCT04796688 | B-cell lymphoma; ALL; CLL | CD19 | Phase 1 | Recruiting |
| NCT04796675 | B-cell lymphoma; ALL; CLL | CD19 | Phase 1 | Recruiting |
| NCT04639739 | B-cell lymphoma | CD19 | Early Phase 1 | Unknown |
| NCT04887012 | B-cell lymphoma | CD19 | Phase 1 | Recruiting |
| NCT05020678 | B-cell lymphoma; B-cell ALL | CD19 | Phase 1 | Recruiting |
| NCT02892695 | ALL; CLL; Follicular lymphoma | CD19 | Phase 1/2 | Unknown |
| NCT03824964 | B-cell lymphoma | CD19/CD22 | Early Phase 1 | Unknown |
| NCT03692767 | B-cell lymphoma | CD22 | Early Phase 1 | Unknown |
| NCT05215015 | AML | CD33/CLL1 | Early Phase 1 | Recruiting |
| NCT05008575 | AML | CD33 | Phase 1 | Recruiting |
| NCT02944162 | AML | CD33 | Phase 1/2 | Unknown |
| NCT02944162 | AML | CD33 | Phase 1/2 | Unknown |
| NCT05247957 | AML | NKG2DL | Phase 1 | Recruiting |
| NCT04623944 | AML; MDS | NKG2DL | Phase 1 | Recruiting |
| NCT05213195 | Metastatic colorectal cancer | NKG2DL | Phase 1 | Recruiting |
| NCT05248048 | Metastatic colorectal cancer | NKG2DL | Early Phase 1 | Recruiting |
| NCT03415100 | Metastatic solid tumors | NKG2DL | Phase 1 | Unknown |
| NCT03940820 | Solid tumors | ROBO1 | Phase 1/2 | Recruiting |
| NCT03931720 | Malignant tumors | ROBO1 | Phase 1/2 | Recruiting |
| NCT03941457 | Pancreatic cancer | ROBO1 | Phase 1/2 | Recruiting |
| NCT05008536 | Multiple myeloma | BCMA | Early Phase 1 | Recruiting |
| NCT03940833 | Multiple myeloma | BCMA | Phase 1/2 | Recruiting |
| NCT03692637 | Epithelial ovarian cancer | Mesothelin | Early Phase 1 | Unknown |
| NCT03692663 | Castration-resistant prostate cancer; | PSMA | Early Phase 1 | Recruiting |
| NCT04847466 | Gastroesophageal junction cancers; Advanced head and neck cancer |
PD-L1 | Phase 2 | Recruiting |
| NCT05194709 | Solid tumors | 5T4 or TPBG | Early Phase 1 | Recruiting |
- Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCMA, B-cell major antigen; CD, cluster of differentiation; CLL, chronic lymphocytic leukemia; CLL1, C-type lectin-like molecule-1; DLBCL, diffuse large B-cell lymphoma; MDS, myelodysplastic syndromes; NHL, non-Hodgkin's lymphoma; NKG2DL, natural killer group 2 member D ligand; PD-L1, programmed death ligand-1; PSMA, prostate-specific major antigen; ROBO1, roundabout guidance receptor 1; TPBG, trophoblast glycoprotein.
NK cells are innate lymphocytes that possess substantial cytolytic activity against virus-infected cells and tumor cells.17 Human NK cells are characterized as CD3−CD56+ cells, and constitute 5%–15% of peripheral blood mononuclear cells (PBMCs).18 According to the different expression levels of CD56, NK cells can be further divided into two subtypes, CD56dim and CD56high NK cells.19 Unlike T cells and B cells, NK cells do not require prior antigens exposure and the engagement of antibodies to mediate antitumor immunity, making them ideal targets for cellular engineering to develop antitumor immunotherapies.20 The effector responses and self-tolerance of NK cells mainly depend on the integration of a variety of activating receptors and inhibitory receptors.21 Major NK cell activation receptors include natural cytotoxicity receptors (NCRs, such as NKp30, NKp40, NKp44, and NKp46), NKG2D, NKG2C, and the Fc receptor CD16.22 In parallel, the inhibitory receptors provide self-tolerance and negative feedback signals. Inhibitory killer Ig-like receptors (KIRs) recognize class I human leukocyte antigen molecules (HLA-A, HLA-B, and HLA-C), whereas NKG2A can bind to HLA-E.23, 24 The recognition of tumor cells by NK cells is based on their unique ability to target and attack cells with low expression levels of MHC class I molecules on the cell surface. The natural antitumor effects of NK cells enable NK cell-based immunotherapeutic strategies. Not surprisingly, CAR-NK cell therapies have recently moved into the spotlight as an alternative cellular immunotherapeutic strategy and can be engineered to target diverse antigens. Unlike CAR-T cell therapy, CAR-NK cells can be derived from allogeneic donors and make it possible to produce “off-the-shelf” living therapeutics. Compared to CAR-T cells, which are mostly effective toward hematological malignancies, CAR-NK cells have shown equally encouraging therapeutic efficacy in solid tumors with enhanced infiltration into the TME.15, 25 Furthermore, CAR-NK cells can evade the resistant TME and achieve potent and persistent antitumor response. Given that engineered cells also possess the natural cytotoxic properties intrinsic to NK cells without the need for CARs (Figure 2), CAR-NK cells can mediate cytotoxic activity in both CAR-independent and CAR-dependent manners to maximize the tumor killing capability.25
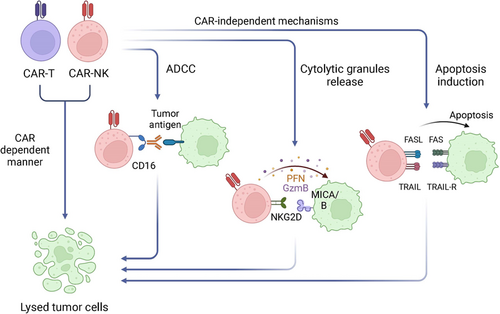
Compared to engineered T-cells, adoptive NK cell-based therapy has several notable advantages. First, NK cells possess pleiotropic cytotoxicity effects, including the release of cytotoxic granules containing perforin and granzymes, secretion of cytokines, and active participation in antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by CD16 receptor.13, 26, 27 For example, activated NK cells can destroy cancer cells by direct cell cytotoxicity via the release of perforin and granzymes and/or by generating proinflammatory cytokines that further attract macrophages and dendritic cells into tumor sites.27-29 Additionally, CAR-NK cells can be engineered with different costimulatory domains (such as 2B4) to enhance costimulatory specificity with regard to NK cell signaling, thereby leading to more rapid proliferation and higher antitumor efficacy.30 Second, NK cells use similar killing mechanisms as CD8+ cytotoxic T-lymphocytes (CTLs) to target and destroy malignant or virus-infected cells yet possess different target recognition machinery. CTLs are activated by T-cell receptors (TCRs) as part of the adaptive immune response, whereas NK cells receive activating and inhibitory signals by their germline-encoded receptor repertoire.14 Because NK cells do not require human leukocyte antigen (HLA) compatibility or antigen recognition to mount an immune response, CAR-NK cells possess a reduced risk of GvHD from the allogeneic transfer of CAR-NK cells from donors.31 Third, CAR-NK cells possess a better safety profile, with CRS and neurotoxicity rarely observed in allogeneic CAR-NK cells in both preclinical studies and clinical trials. Activated NK cells typically secrete IFN-γ and GM-CSF,32 while activated CAR-T cells tend to elicit the production of copious amounts of IL-1α, IL-2, IL-6, TNF-α, IL-8, and IL-10, some of which are highly correlated with CRS.33 These desirable features of CAR-NK cells could substantially mitigate side effects, reduce healthcare costs, and improve cancer patient outcomes.
2 CAR-NK CELL GENERATION
Autologous CAR-NK cell infusion is the safest approach for adoptive cell therapy because it relies on the patient's own blood as the cell source and obviates the need for immunosuppressive pretreatment of the patient. However, the reduced risk of alloreactivity inherent to NK cells allows CAR-NK cells to be generated from multiple allogeneic sources (Figure 3), including PBMCs, hematopoietic progenitor cells (HPCs), umbilical cord blood (UCB), NK-92 cell lines, and induced pluripotent stem cells (iPSCs).25, 34 This flexibility allows CAR-NK cells to be generated without the personalized, patient-specific production that CAR-T cell therapy often requires. Therefore, CAR-NK cells hold great promise to be manufactured as “off-the-shelf” products for on-demand and generic use in cancer patients with substantially reduced costs.
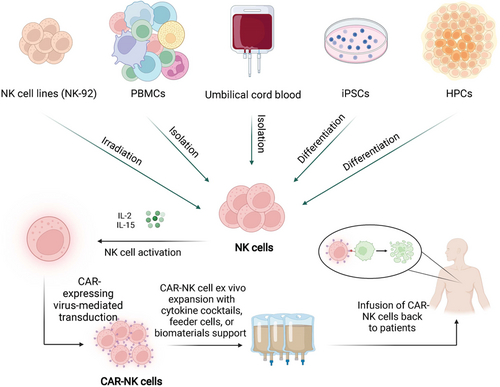
The gene encoding CAR is often introduced into peripheral blood-isolated (PB-NK) or stem cell-induced NK cells or NK-like cell lines via viral transduction using lentivirus or retrovirus, mRNA electroporation, or Sleeping Beauty transposon. CAR-expressing NK cells are then expanded in NK cell-specific expansion media with expensive cytokines for GMP-grade clinical application until adequate cell numbers are attained. PB-NK cells can be most easily obtained but have relatively low transduction efficiency and limited expansion capacity.35 Stem cell-derived products offer a rich source of CAR-NK cells, and enable the generation of standardized generic therapies for any patient irrespective of the HLA haplotype. For instance, UCB stem cell-derived NK cells used in the first clinical trials of CAR-NK cell therapy have great proliferative competence. They can be expanded by 2000-fold ex vivo with over 90% purity for the functional NK cell population.36, 37 However, UCB-NK cells might suffer from condensed cytotoxicity due to their immature nature, and iPSC-derived NK cells are claimed to exhibit stronger anticancer activity than UCB-NK and remain as effective as PB-NK cells in several mouse models of cancer.38, 39
Clonal cell lines with NK cell-like properties, including NK-92, YT, and KHYG-1, could serve as alternative sources for CAR-NK cells, with NK-92 most extensively used in both preclinical and clinical applications. The NK92 cells are easier for genetic engineering and ex vivo expansion with a doubling time of approximately 24−36 h. However, because this cell line is derived from a 50-year-old white male diagnosed with rapidly progressive non-Hodgkin's lymphoma, NK92 cells have to be lethally irradiated before infusion back to the patient, which tends to limit the in vivo persistence of the engineered cells and impair durable clinical efficacy.40, 41 This obstacle has been partially tackled via the expression of a high-affinity CD16 variant (haNK) to boost the effector function.42 The clinical efficacy of this novel approach, along with the combined use of cancer vaccines or other agents, is yet to be revealed by ongoing clinical trials (NCT03387085, NCT03853317, NCT03387111, and NCT03586869).
In general, CAR-NK cells have safer profiles than clinically approved CAR-T cells, and elicit minimal CRS with decreased or negligible neurotoxicity. The decrease in neurotoxicity and CRS can be explained by the different cytokine production patterns between NK and T cells. CAR-NK cells mainly secrete IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas CAR-T cells predominantly secrete proinflammatory interleukins (e.g., IL-6) and TNF-α, which are highly associated with CRS and neurotoxicity.13, 34 Additionally, because of the limited lifespan of CAR-NK cells circulating within the body, CAR-NK cells have a reduced risk of on-target/off-target toxicity to normal tissues. The addition of tumor antigen-specific adapter molecules (AMs) and CAR immune cells that are specific for these AMs leads to a more targeted, precise, and temporally restricted immune response.43 As a result, multiple antigens can be targeted simultaneously by using CAR-NK cells. Collectively, advances in gene manipulation and bioengineering techniques have resulted in CAR-NK cell therapy with strong antitumor activity and mostly nontoxic effects on normal tissues after infusion into patients.16
3 THERAPEUTIC EXPLORATION WITH CAR-NK CELL THERAPY
Following the huge clinical successes of CAR-T therapies, CAR-NK cell-based therapies have been increasingly tested and optimized in various mouse models of tumors, as well as in clinical trials (Table 1). However, due to the limited progress of clinical trials, biosafety and efficacy of CAR engineered NK cell therapies in human are yet to be rigorously examined in the clinical setting. We present herein some representative preclinical achievements by using CAR-NK cells derived from different cell sources.
3.1 Preclinical studies in hematological malignancies
CAR-NK cell therapies have been widely used to target CD19+ B-cell malignancies in various mouse models. Romanski et al.44 transduced NK-92 cells with anti-CD19 CAR and demonstrated that the CD19 CAR-NK cells could specifically lyse CD19-expressing B-precursor leukemia. Liu et al. engineered cord blood-originated NK cells with CD19-CAR, IL-15, and an inducible caspase-9 suicide gene. Both in vitro and in vivo studies showed robust efficacy of engineered NK-CAR cells to eliminate tumors. By administrating a dimerizing agent to trigger apoptosis, CAR-NK cells can be further conditionally eradicated, thereby adding an additional safety switch to mitigate potential side effects.45 Muller et al.46 explored anti-CD20 NK cells and showed efficient cytotoxicity toward NK cell-resistant lymphoma and leukemia cells. Furthermore, Boissel et al.47 have shown that CD20 CAR-NK cells display exceptional cytotoxicity toward primary chronic lymphocytic leukemia cells compared to anti-CD20 mediated ADCC.
To kill T-cell malignancies, CARs targeting CD3 and CD5 have been designed and engineered into NK-92 cells. Chen et al.48 developed CD3 CAR-NK cells and demonstrated their effectiveness in cytotoxicity toward CD3+ human peripheral T-cell lymphomas. In addition, CD5 CAR-NK-92 cells were generated to eradicate T-cell acute lymphoblastic leukemia (T-ALL), as well as a variety of T-lineage leukemia cell lines. In xenograft T-ALL models, CD5 CAR-NK-92 cells significantly suppressed tumor growth and improved the overall survival of tumor-bearing mice.49 CAR-NK cell therapies also showed their benefits in other types of hematological neoplasms, including but not limited to acute myeloid leukemia, lymphoma, and multiple myeloma.50-53 Additionally, when combined with other monoclonal antibody (mAb)-based therapies (e.g., anti-CD38 mAb), FT576 NK cells exhibited enhanced cytotoxicity, prevention of antigen escape, durable persistence, and avoidance of self-fratricide.54
3.2 Preclinical studies in solid tumors
Considering the intrinsic antitumor properties of NK cells (e.g., killing cancer cells by the “missing-self” mechanism, cytolytic ability, and ADCC), CAR-NK therapies may possess unique strengths to fight against solid tumors.55 Although NK cell activities might be partially impaired in TME, accumulating evidence suggests that NK cells can effectively infiltrate solid tumors and maintain cytotoxicity against cancer cells.56-59 The preclinical exploration of CAR-NK cell therapy for solid tumors have shown promising efficacy. The human epidermal growth factor receptor (EGFR) is involved in mediating the activity and proliferation of cells, which has been identified as a critical therapeutic target in various malignancies.60, 61 It has been shown that EGFP-engineered NK cells exhibit improved cytolytic capability. Han et al. engineered wtEGFR- and EGFRvIII-targeting CAR-NK-92 cells, which were shown to display strong tumor-killing efficacy when cocultured with glioblastoma. Intracranial administration of CAR-modified NK cells in xenograft mouse models significantly suppressed tumor growth.62 Furthermore, EGFPR-CAR-NK cells were designed to treat triple-negative breast cancer (TNBC), presenting robust efficiency in curtailing tumor growth in both human breast cancer cell-derived xenograft and patient-derived xenograft (PDX) mouse models.63
Ganglioside GD2 is a well-established antigen for neuroblastoma (NB) immunotherapy. Mitwasi et al.64 built a modular universal CAR (UniCAR) platform and redirected NK-92 cells to efficiently target GD2+ cancer cells. Importantly, the UniCAR system can be flexibly modified to target other tumor-specific antigens. Folate receptor α (FRα) is overexpressed in various solid tumors, such as ovarian, lung, and pancreatic ductal adenocarcinoma (PDAC), and thus becomes a promising anticancer target.65 NK-92 cells expressing FRα-CAR-28BBζ showed antigen-specific cytotoxicity and lower antigen-induced apoptosis toward ovarian cancer.66 Lee et al.67 reprogrammed CAR-NK cells to target FRα carrying an apoptosis ligand and demonstrated selective cytotoxicity to PDAC. More preclinical testing in animal models is anticipated in the forthcoming years.
Despite the safety and promising preclinical efficacy of allogeneic CAR-NK cells studies, CAR-NK cell-based clinical research is still in its infancy compared to the fully fledged CAR-T cell technology. Over two dozen studies have been registered on Clinicaltrials.gov (see examples in Table 1), with the majority targeting hematological malignancies by taking advantage of CD19, CD22, BCMA, and CD33 antigens. In trials with solid tumors, targeted antigens include prostate-specific membrane antigen (PSMA), mesothelin, roundabout guidance receptor 1 (ROBO1), and natural killer group 2 member D ligand (NKG2DL). CAR-NK cell therapy will likely gain huge momentum by reaching the milestone of FDA approval for cancer treatment in the near future.
4 OVERCOMING ROADBLOCKS IN CAR-NK CELL THERAPY
Despite all the aforementioned encouraging advances, several roadblocks have to be addressed to accelerate the clinical translation of NK cell-based therapy. Recent efforts have been predominantly directed to reprogram and modify NK cells to maximize their expansion and persistence, promote their trafficking or infiltration, and combat the immunosuppressive TME.
4.1 Boosting expansion ex vivo
Because a single infusion in the patient requires a large number of engineered CAR-NK cells, approaches to reduce the cost and time associated with the ex vivo expansion of NK cells are of urgent clinical interest. To facilitate the expansion of NK cells ex vivo during the preparation of CAR-NK cells, iPSC-derived NK cells have been generated and tested in multiple preclinical studies. A recent study has demonstrated that iPSC-NK cells retain similar cytokine secretion and cytotoxic capacity as commonly used PB-NK cells.68 More importantly, they are capable of efficient infiltration into tumor spheroids in vitro and engaging other effector immune cells in vivo. In addition to optimizing the cocktail of cytokines (IL-2 and IL-15) used to activate and expand NK cells ex vivo, researchers have explored other creative strategies as well. One such approach involves the use of a K562 feeder cell line that expresses 4-1BBL and IL-21 on the cell surface, which leads to a nearly 48,000-fold expansion of functional NK cells within one and a half months.69-71 Most impressively, these expanded cells showed no signs of cell senescence nor compromised antitumor cytotoxicity, and turned out to be relatively safe when injected back into patients.72, 73 In parallel, biomaterials have been explored to support the expansion of NK cells. Graphene oxide-based nanomaterials with surface modifications of CD16 have been shown to effectively activate NK cells.74 In another study, a three-dimensional engineered hyaluronic acid-based polymeric scaffold (designated 3D-ENHANCE) with macro-porous structures was used to boost the ex vivo expansion of human NK cells.75
4.2 Increasing persistence and survival in vivo
One potential caveat associated with CAR-NK cell therapy is the lack of durable persistence and survival in vivo. NK cells infused into humans can only be detected in the circulation by flow cytometry for only a few weeks.17, 76 On the positive side, this might make the adoptive cell therapy safe because treatment discontinuation can be achieved easily to reduce potential side effects. However, it could limit the efficacy and necessitate injections of multiple doses to cause cost increase and inconvenience. Several approaches have been explored to address this issue. For example, systematic administration of IL-15 or similar agonists (N-803 with IL-15 bound to IL-15Rα-IgG1-Fc fusion) has been tested in clinical trials to boost adoptive cell therapy persistence and survival in patients.77 In parallel, CAR-NK cells derived from stem cells have been engineered to co-express transgenes encoding cytokines (e.g., IL-15) or their complexes with cognate receptors (e.g., IL-15-IL15Rα), via constitutive expression in the cells or in the form of membrane-bound cytokines.45, 78 Another approach involves the use of cytokine cocktails to induce NK cell differentiation into a memory-like state.79-82 CAR-NK cells preactivated by IL-12/IL-15/IL-18 showed improved potency against NK-resistant B-cell lymphoma.81 According to a recent Phase 1 clinical trial results, cytokine-induced memory-like (CIML) NK cells were found to cause up to 50-fold expansion in vivo that could last for up to 6 months.83 Most recently, efforts have also been made to genetically modify the negative regulator of cytokine or cytokine checkpoint signaling, such as depleting the cytokine-inducible Src homology 2-containing protein, in iPSC-NK or UCB-NK cells, thereby reprogramming metabolic fitness to increase overall persistence and promote tumor clearance in mouse models of leukemia and lymphoma.84, 85
4.3 Promoting trafficking and infiltration
Effective trafficking and infiltration of NK cells into the tumor sites is often hampered by the downregulation of genes involved in the chemokine networks and/or lack of strong chemotactic signaling in the tumor sites. Chemokine network engineering or modulation in both NK cells (e.g., CXCR1/2/3/4, CCR5, CCR7, and CX3CR1) and the TME (with IFN-γ and IL-12) have been tested by multiple groups to increase NK cell trafficking/homing to lymph nodes, and further promote immune cell infiltration into tumors to boost tumor killing.86-94 For example, overexpression of CXCR1 in NKG2D-CAR-NK cells was found to enhance the in vivo cytotoxicity against ovarian cancer, which is known to secrete IL-8 as the ligand of CXCR1.87 Similarly, CXCR4 overexpression in EGFRvIII-CAR-NK cells was shown to facilitate the infiltration of engineered NK cells into tumor sites and improve the antitumor efficacy toward glioblastoma secreting CXCL12 (a CXCR4 ligand) in mouse models.92 Furthermore, CXCR4 and CCR7 were both overexpressed in NK-92 cells to improve migration toward the tumor cells and ameliorate human colon cancer in rodent models.91 In parallel, intratumoral injection of IFN-γ has been demonstrated to induce the upregulation of CXCL9/10/11 within the TME, thereby creating strong chemotactic signals to augment NK cells infiltration in both mouse and rabbit models.93, 95 Despite these encouraging results from preclinical studies, chemokine- and/or chemokine receptor-engineered CAR-NK cells are yet to be rigorously tested in clinical trials.
4.4 Combating suppressive TME
The presence of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), as well as suppressive cytokines and metabolic factors (e.g., IL-10, transformative growth factor-beta (TGFβ), indoleamine 2,3-dioxygenase, prostaglandin E3, hypoxia, low pH and nutrient deficiency) within solid tumors create an immunosuppressive microenvironment to inhibit the effector function of NK and CD8+ T cells.96-100 For instance, TGFβ overproduction within TME is known to suppress functional/mature NK cell recruitment, inhibit perforin secretion, and downregulate genes that are important for NK cell activation (such as NKG2D).101 To counteract the immunosuppressive activity of TGFβ, researchers have explored at least two approaches to render NK cells resistant to TGFβ. First, a high-affinity dominant-negative TGFβ receptor 2 (TGFβR2) incapable of signal transduction was expressed in UCB-NK, which retained high cytotoxicity toward leukemia and glioblastoma cell lines even in the presence of TGFβ.102 Second, genome editing was applied to deplete TGFβ receptor 2 (TGFβR2) in NK cells isolated from human patients with myelodysplastic syndromes (MDS), thereby making them insert to TGFβ and retaining strong effector function.103 The in vivo performance of TGFβR2-KO NK cells is still under evaluation by using a xenograft model of leukemia. A similar genome editing approach can be extended to remove negative receptors or checkpoint genes in NK cells to improve their tumor-killing capacity. In addition, the combined use of CAR-NK with FDA-approved immune checkpoint blockers (anti-PD1/PD-L1 or anti-CTLA4 biologics) will likely maximize the effector function of engineered NK cells within TME.104, 105
5 CONCLUSION
Compared to T cells, NK cells are important innate immune cells of the body, which can kill tumor cells without pre-sensitization. The accelerated research progress of NK cell-based therapy shows a promising efficacy on solid tumors. CAR-NK is expected to be one of the most anticipated engineered cell therapies following the recent success of CAR-T cell therapy. In addition to directly eradicating tumors expressing the target antigen, CAR-NK cells retain their natural cytotoxicity toward tumor cells with the loss of surface antigens, making it possible to overcome tumor antigen escape that is often encountered during CAR-T cell therapy. Because NK cells express CD16 (also known as FcγRIIIa) on their surface, they can interact with the Fc fragment of antibodies to trigger ADCC. CAR-NK cells can thus be further combined with existing mAb-based therapies to prevent tumor escape and boost the tumor-killing efficacy. These properties indicate that a wider range of target antigens for CAR-NK cells can be exploited in parallel. Creative immune-engineering and powerful genome editing techniques are likely to overcome many hurdles facing the expansion, persistence, trafficking, infiltration, and survival of CAR-NK cells. In aggregate, CAR-NK, as a relatively new addition to cellular immunotherapy, holds great promise to become a first-line strategy in cancer treatment and, therefore, has high translational values in the clinic to directly benefit cancer patients.
AUTHOR CONTRIBUTIONS
Yubin Zhou conceived the idea and supervised the work. Yubin Zhou, Siyao Liu, Kaycee Nguyen, and Dongyong Park drafted the manuscript with inputs and further editing from all other authors.
ACKNOWLEDGMENTS
This study was supported by the NIH grants (R01CA232017 to Y. Z.), the Cancer Prevention and Research Institute of Texas grant (CPRIT; RP210070 to Y. Z.), and by the Welch Foundation grant (BE-1913-20220331 to Y. Z.).
CONFLICT OF INTEREST
Professor Yubin Zhou is a member of Chronic Diseases and Translational Medicine editorial board and is not involved in the peer review process of this article. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Data available upon request.