Emerging role of IRE1α in vascular diseases
Abstract
A mounting body of evidence suggests that the endoplasmic reticulum stress and the unfolded protein response are involved in the underlying mechanisms responsible for vascular diseases. Inositol-requiring protein 1α (IRE1α), the most ancient branch among the UPR-related signaling pathways, can possess both serine/threonine kinase and endoribonuclease (RNase) activity and can perform physiological and pathological functions. The IRE1α-signaling pathway plays a critical role in the pathology of various vascular diseases. In this review, we provide a general overview of the physiological function of IRE1α and its pathophysiological role in vascular diseases.
1 INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide. According to the World Health Organization, 32% of all global deaths worldwide are due to CVD.1 Vascular diseases, including atherosclerosis, systemic and pulmonary hypertension, neointimal hyperplasia, vascular calcification, aneurysms, and other disorders, are common risk factors for overall mortality. The pathology of vascular diseases is closely related to the dysfunction of the vasculature and vascular cells. The arterial wall contains three layers: the intima, media, and adventitia. The intima, the innermost layer, is a monolayer of endothelial cells (ECs), which serve as the primary responder in the regulation of vessel tone, permeability, and interaction with circulating molecules.2 The media contain predominantly vascular smooth muscle cells (VSMCs), which are crucial for the regulation of vessel tone, the blood stream, and blood pressure.3 The adventitia, the outer layer of the artery, is composed of numerous fibroblasts and myofibroblasts, and a small number of VSMCs, adipocytes, pericytes, and inflammatory cells.4 Under pathological conditions, these cells coordinate together and positively participate in the progression of vascular diseases. The development of vascular diseases depends on many factors, such as inflammation,5, 6 oxidative stress,7, 8 DNA damage,9, 10 endoplasmic reticulum (ER) stress,11, 12 and metabolic reprogramming.3 Among these factors, inositol-requiring protein 1α (IRE1α) plays an essential role in multiple mechanisms responsible for vascular diseases. In this review, we summarize the role of IRE1α in vascular diseases.
2 THE LOCALIZATION, STRUCTURE, AND SIGNALING PATHWAY OF IRE1α
To fully understand the role of IRE1α in vascular disease, we will provide a brief overview of the localization, structure, and signaling pathway of IRE1α.
2.1 ER stress and the unfolded protein response (UPR)
In eukaryotic cells, the ER is the largest membrane-bound organelle and performs pivotal cellular functions such as membrane biogenesis, protein synthesis and modification, lipid synthesis and trafficking, and calcium storage and secretion.13, 14 The ER is essential for proteostasis, as more than one-third of cellular proteins are synthesized and folded in the ER. Under a wide range of pathophysiological conditions, the accumulation of unfolded or misfolded proteins overwhelms the protein folding capability of the ER, leading to ER stress and the activation of an adaptive response called the UPR15 to preserve proteostasis, which includes protein overaccumulation, improved protein folding, and the degradation of misfolded proteins.11 Three main signaling branches mediate the UPR, known as IRE1 (α and β), activating transcription factor 6α (ATF6α) and ATF6β, and protein kinase RNA (PKR)-like ER kinase (PERK). Under severe or consistent ER stress, homeostasis is disrupted in the ER, and overactivation of the UPR results in the activation of cell death programs.13, 16 Work over the last 3 decades has pinpointed the UPR as a driving force for the progression of a variety of diseases, including cancer,17-19 central nervous system diseases,20, 21 metabolic diseases,22-24 and CVD.11
2.2 The structure of IRE1α
IRE1 is the most ancient branch of the UPR-related signaling pathway and is evolutionarily conserved from yeast to humans.25 IRE1 has two isoforms: IRE1α (encoded by the gene ER to nucleus signaling 1 (Ern1)) and IRE1β (encoded by the gene Ern2). IRE1α is abundant and prevalent in almost all tissues, while IRE1β is expressed only in airway mucous cells and intestinal epithelial cells.26 IRE1 consists of two domains: the N-terminal, ER luminal domain, and the C-terminal cytoplasmic region, serine/threonine kinase and endoribonuclease (RNase) domains (Figure 1).27 The LD is used to sense unfolded or misfolded proteins and monitor ER homeostasis, and the C-terminal domain triggers the UPR.27 The unfolded protein can directly bind to the LD, which triggers IRE1 oligomerization and allows trans-autophosphorylation of the kinase domain28. The only known substrate of the kinase is IRE1 itself, and the RNase of IRE1 is regulated by the intrinsic kinase module of IRE1.28
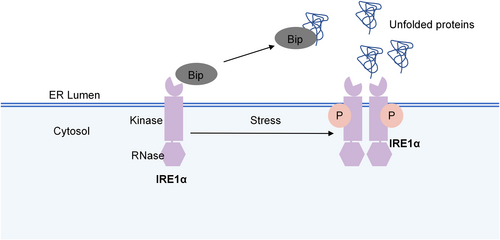
The structure of IRE1α. IRE1α consist of two domains: the endoplasmic reticulum luminal domain, and serine/threonine kinase and endoribonuclease (RNase) domains. In resting conditions, IRE1α binds to Bip/GRP78. Upon stress, IRE1α disintegrates with Bip/GRP78 and activated via autophosphorylation and dimerization/oligomerization.
2.3 The activation of IRE1α
ER plays a critical role in protein and lipid synthesis, and regulate lipid and glucose metabolism.29 As a branch of UPR, IRE1α has been reported to be activated by several types of signals, including nutrients, metabolisms, and hormones.13 Glucose and lipid have been considered as stressors for activating IRE1α-signaling pathway. It has been well-documented that high glucose stimulated the hyperactivation and phosphorylation of IRE1α in pancreatic β cells,30, 31 and the prolonged activation of IRE1α contributes to β -cell apoptosis in type 1 diabetes.32 Moreover, high glucose activated IRE1α-signaling pathway in retinal muller cells and renal tubular epithelial cells, which further results in diabetic retinopathy and diabetic nephropathy, respectively.33, 34 In turn, the highly phosphorylated IRE1α in the liver drives hyperglycemia under metabolic ER stress.35 Lipid also stimulates the activation of IRE1α. IRE1α could be activated by lipid independently of its lumenal unfolded protein stress-sensing domain.36 Under metabolic ER stress conditions, activation of IRE1α underlies the dysregulation of thermogenic fat in obese mice.37 Besides, IRE1α has been reported to be activated in both human and rodent obese adipose tissue.38, 39 In addition, high fat diet induced the activation of IRE1α in macrophages, hepatocytes, and ECs.40-43
Numerous studies have shown that IRE1α could be activated by metabolic hormones. Insulin is an anabolic hormone, which is a participant in protein synthesis and lipogenesis, and results in increased load of protein folding in the ER.13 The phosphorylation of IRE1α was observed in mice with hyperinsulinemia and insulin resistance induced by high fat diet.44 Targeting IRE1α improves insulin resistance and glucose intolerance in obese mice.39 Besides, mice with β cell-specific deletion of IRE1α exhibited a diabetic phenotype, with less insulin secretion and elevated blood glucose level after feeding.45 In addition, IRE1α is metabolically activated by glucagon in the liver, and glucagon or epinephrine could stimulate protein kinase A, which further phosphorylated IRE1α at Ser724.35
2.4 The signaling pathways of IRE1α
Under resting conditions, IRE1α binds to Bip/GRP78 in the ER.24 Upon stress, IRE1α disintegrates with Bip/GRP78 and is activated via autophosphorylation and dimerization/oligomerization, leading to RNase activation .13 In mammalian cells, the active RNase cleaves 26 nucleotides from the unspliced form of X-box binding protein-1 (XBP1u) mRNA and produces the spliced form of XBP1 (XBP1s) mRNA.46 XBP1u contains a nuclear C-terminus called hydrophobic region 2 and a degradation domain, while the C-terminus of XBP1s has a transcriptional activation domain.46 This difference makes XBP1s a crucial transcription factor, which could translocate into the nucleus and initiate transcriptional processes, thus participating in ER biogenesis, protein folding, and ER-associated degradation.24 After being translocated to the nucleus, XBP1s binds to ER stress-responsive element I and promotes the transcription of mesencephalic astrocyte-derived neurotrophic factor (MANF).47 Inactivation of MANF contributes to the upregulation of a pro-apoptotic component of the UPR, C/EBP-homologous protein (CHOP).48 In addition, IRE1α cleaves a subset of ER-associated mRNAs, resulting in their degradation through an activity named regulated IRE1-dependent decay (RIDD) (Figure 2).
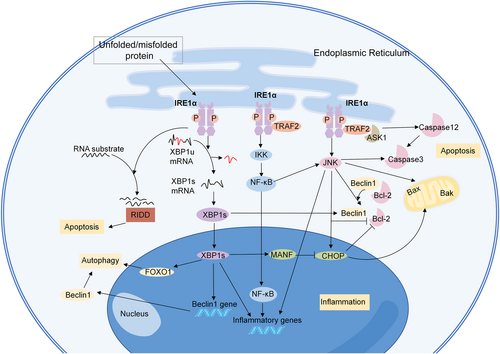
The IRE1α-signaling pathway. The active RNase cleaves 26 nucleotides from unspliced form of XBP1 (XBP1u) mRNA and produce the spliced form of XBP1 (XBP1s) mRNA. XBP1s is a crucial transcription factor, which could translocate into the nucleus and initiate the transcriptional process. IRE1α also cleaves a subset of ER-associated mRNAs. IRE1α activates several signaling pathways through inflammation, apoptosis, and autophagy. ASK1, apoptosis-signaling kinase 1; CHOP, C/EBP-homologous protein; FOXO1, Forkhead box O1; IKK, IκB kinase; JNK, c-Jun NH2-terminal kinase; LD, luminal domain; MANF, mesencephalic astrocyte-derived neurotrophic factor; NF-κB, nuclear factor-kappa B; RIDD, regulated IRE1-dependent decay; TRAF2, tumor necrosis factor receptor associated factor 2; XBP1s, spliced form of X-box binding protein-1; and XBP1u, unspliced form of X-box binding protein-1.
IRE1α is associated with well-known risk factors for vascular diseases, such as inflammation, autophagy, and apoptosis. IRE1α has been shown to trigger inflammation in vascular diseases. The dimerization of IRE1α stimulates its protein kinase domain, and induces the binding of tumor necrosis factor receptor associated factor 2 (TRAF2) to the IRE1α complex.49 This thereby directly activates the apoptosis-signaling kinase 1 (ASK1) and IκB kinase (IKK) pathways, and subsequently activates the c-Jun NH2-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB) pathways.49-51 IKK is a crucial kinase that responds to inflammatory stimuli and can increase the activity of XBP1s.52 IRE1α can promote inflammatory cytokine generation through the NF-κB pathway.53 IRE1α/NF-κB can activate the JNK-signaling pathway, and therefore induce the transcription of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8.54 IRE1α also regulates the expression of proinflammatory cytokine genes through the activation of XBP1s and glycogen synthase kinase (GSK)-3β.55
IRE1α can promote both apoptosis and autophagy, which are essential processes that mediate vascular diseases.56 IRE1α has been reported to be the main switch between these autophagy and apoptosis.57 Some researchers believe that the real cell autophagic-signaling pathway induced by ER-stress involves IRE1α, not PERK or ATF6, as IRE1α is essential for the autophagosome formation and LC3-II conversion.57-59 As we have mentioned above, upon ER-stress, IRE1α binds to TRAF2 and ASK1, and the formation of the IRE1α-TRAF2-ASK1 complex activates the JNK pathway, and mediates ER stress-induced cell autophagy.58, 60 Active JNK mediates Bcl-2 phosphorylation and disassociates the Beclin-1/Bcl-2 complex, thus releasing free Beclin-1.61 The IRE1α-JNK pathway also mediates autophagic cell death through the CHOP-signaling pathway.62, 63 The IRE1α/XBP1s pathway also regulates autophagy through Beclin-1.64, 65 Chromatin immunoprecipitation assays revealed that XBP1s can directly bind to the promoter of Beclin-1 at the region from nt −537 to −755.64 XBP1s also triggers autophagy via the Forkhead Box O1 (FoxO1) pathway, while XBP1u serves as a negative regulator because it recruits FoxO1 to the 20S proteasome and promotes FoxO1 degradation.66, 67
In the early stage of ER-stress, the phosphorylation of Bcl-2 mediated by JNK triggers autophagy, while sustained JNK activation induces apoptosis.68 The IRE1α-TRAF2-JNK axis enhances pro-apoptotic Bax and Bak activity and represses anti-apoptotic Bcl-2 activity.69-72 In addition, the TRAF2 not only facilitates the activation of caspase-3 and promotes apoptosis through the JNK pathway73, 74 but also directly activates caspase-12, which is specifically localized at the ER and can activate cleaved-caspase-3.57, 73 IRE1α can induce apoptosis through CHOP, and CHOP downregulates Bcl-2 and Mcl-1, and releases Bax to form pores in the mitochondrial outer membrane, which subsequently activates caspase3 and induces apoptosis.54, 73 In addition, IRE1α mediates ER stress-induced apoptosis through the RIDD pathway.75
3 IRE1 IN VASCULAR DISEASES
3.1 Atherosclerosis
Atherosclerosis is the most common vascular disease worldwide.76-78 Chronic inflammation, imbalanced lipid metabolism, and an aberrant immune system response contribute to atherosclerosis through acting on vascular cells.79, 80 The ECs, macrophages, monocytes, and foam cells are involved in atherosclerotic lesion initiation.81 The risk factors for atherosclerosis, such as hyperhomocysteinaemia, oxidative stress, reactive nitrogen species, and free cholesterol accumulation in macrophages, contribute to IRE1α activation, which in turn promotes atherosclerosis.11
Phosphorylation of IRE1α is observed in human atherosclerotic lesions but is not detected in normal arteries.82 Moreover, the expression of p-IRE1α was greater in the cores of advanced carotid atherosclerotic lesions than in those of stable aortic plaques, suggesting a key role for IRE1α in atherogenesis.82 This notion is also supported by the results from rodent studies. The phosphorylation of IRE1α is induced in the aortic intima and atherosclerotic lesions of ApoE−/− mice, and is significantly greater in ApoE−/− mice subjected to 5/6 nephrectomy than in controls.71, 83, 84 Furthermore, XBP1s, the downstream of IRE1α, was abundantly detected at branch points and in atherosclerotic lesions of ApoE−/− mice and spontaneously hypertensive rats (SHRs) fed a cholesterol-enriched diet.65, 85, 86
The initiation of atherosclerosis begins with the EC dysfunction; ECs facilitate the transport of low-density lipoprotein (LDL) into the subendothelial space, and the accumulation of LDL provokes an immune response.87 In the atherosclerotic model of swine, the expression of both p-IRE1α and XBP1 was upregulated in the endothelium from the athero-susceptible arterial region compared with the athero-protected region.88 In addition, overexpression of XBP1s induces atherosclerotic lesions in a thoracic aorta isograft model.85 Numerous studies have reported that the activation of IRE1α in human umbilical vein ECs (HUVECs) and human aortic ECs (HAECs) is associated with risk factors for atherosclerosis, including LDL, homocysteine, and high glucose levels (Figure 3).89-92 In addition, exposure to arsenite, an inducer of atherosclerosis, can activate IRE1α/XBP1s to promote hypoxia inducible factor 1α (HIF1α) accumulation.93 The XBP1s/HIF1α complex further regulates the transcription of angiotensinogen, angiotensin-converting enzyme, and angiotensin II type 1 receptor, which play a role in endothelial dysfunction and inflammatory reactions.93 Inhibition of the IRE1α/XBP1s/HIF1α pathway attenuated arsenite-induced oxidative stress and the proinflammatory response.93
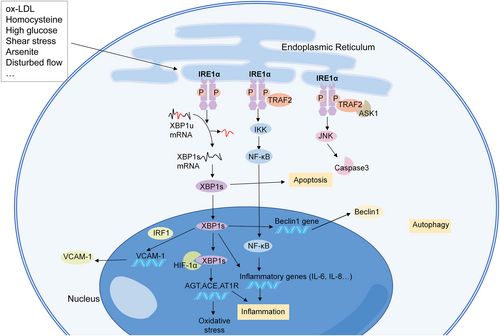
Mechanisms of IRE1α in endothelial cells (ECs) in atherosclerosis. IRE1α can be activated in ECs treated with risk factors for atherosclerosis, including oxidized low-density lipoprotein, homocysteine, high glucose, shear stress, arsenite, and disturbed flow. IRE1α participates in the progression of atherosclerosis through inflammation, apoptosis, and autophagy. ACE, angiotensin-converting enzyme; AGT, angiotensinogen; ASK1, apoptosis-signaling kinase 1; AT1R, angiotensin II type 1 receptor; HIF1α, hypoxia inducible factor 1α; IRF1, interferon regulatory factor 1; IKK, IκB kinase; JNK, c-Jun NH2-terminal kinase; NF-κB, nuclear factor-kappa B; ox-LDL, oxidized low-density lipoprotein; TRAF2, tumor necrosis factor receptor associated factor 2; VCAM-1, vascular cell adhesion molecule-1; XBP1s, spliced form of X-box binding protein-1; and XBP1u, unspliced form of X-box binding protein-1.
EC apoptosis plays a pivotal role in the initiation and progression of atherosclerosis. IRE1α participates in the apoptosis of ECs, which promotes atherosclerotic plaque formation and destabilization.94 The IRE1α-JNK pathway plays a role in the apoptotic effect in oxidized LDL (oxLDL)-treated ECs, and inhibiting the IRE1α/JNK/caspase-3 pathway could protect HUVECs against apoptosis.74, 82 Sustained activation of XBP1s results in HUVEC apoptosis, endothelial denudation, and lesion progression through downregulation of the VE-cadherin and activation of multiple caspases.85 The IRE1α-XBP1s pathway also regulates the autophagy of ECs in atherosclerosis and induces an autophagic response and death in ECs through the transcriptional activation of Beclin-1.64 IRE1 also triggers inflammatory pathways. The IRE1α/NF-κB pathway results in lipopolysaccharide-induced HUVEC injury through the promotion of inflammatory cytokine production.53 In addition, XBP1 is an essential mediator of inflammatory factors in ECs, including IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, and Chemokine (CXC) motif ligand 3 (CXCL3).95
Atherosclerosis tends to occur in arteries with bifurcations, which exhibit turbulent blood flow, where shear stress is low.81, 96 Low shear stress regulates inflammation in HAECs through the activation of XBP1, which together with interferon regulatory factor 1, upregulates the expression of the vascular cell adhesion molecule-1.97 Disturbed flow leads to the disturbances in CLOCK expression and the activation of the IRE1α-XBP1 pathway, which results in endothelial-to-mesenchymal transition and inflammation, and ultimately vulnerable plaque progression.98 In contrast, steady laminar flow can downregulate XBP1s through the phosphoinositide 3-kinase (PI3K)/Akt pathway.99
The macrophages in advanced atherosclerotic lesions contribute to lesion necrosis, plaque rupture, and acute atherothrombotic vascular occlusion.71 Transcriptome analysis revealed that IRE1α regulates the expression of proatherogenic genes in primary mouse bone marrow-derived macrophages (BMDMs).100 In the peritoneal macrophages of ApoE−/− mice with hyperlipidemia, the phosphorylation of IRE1α is observed.101 In vitro, cholesterol can induce the expression of both p-IRE1α and XBP1 in macrophages.71, 72, 102 Stimulation of macrophages with angiotensin II also induces the phosphorylation of IRE1α in a dose- and time-dependent manner.83 In addition, XBP1 splicing is enhanced in oxLDL-induced human leukemic monocytic THP-1 cells and the mouse macrophage-like cell line J774A1103-105 (Figure 4).
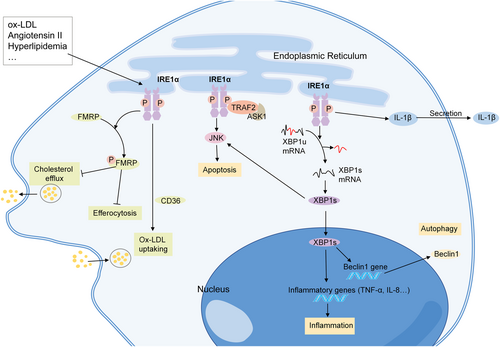
Mechanisms of IRE1α in macrophages in atherosclerosis. IRE1α can be activated in macrophages treated with oxidized low-density lipoprotein and angiotensin II. IRE1α participates in the progression of atherosclerosis through inflammation, apoptosis, and autophagy. IRE1 kinase activation induces Fragile X Mental Retardation protein (FMRP) phosphorylation, and suppresses the macrophage cholesterol efflux and efferocytosis. IRE1α also promotes oxLDL uptaking through CD36. ASK1, apoptosis-signaling kinase 1; FMRP, Fragile X Mental Retardation protein; JNK, c-Jun NH2-terminal kinase; ox-LDL, oxidized low-density lipoprotein; TRAF2, tumor necrosis factor receptor associated factor 2; XBP1s, spliced form of X-box binding protein-1; and XBP1u, unspliced form of X-box binding protein-1.
The apoptosis of macrophages is one of the main consequences of prolonged IRE1α activation. Activated IRE1α in macrophages affects the TRAF2-ASK1-JNK pathway, and the subsequent apoptosis.71, 72 In addition, the activation of the IRE1α-XBP1-JNK axis leads to the apoptosis of foam cells.106 As for XBP1s, Tian et al. revealed that transient overexpression of XBP1s in macrophages induces autophagy through the upregulation of Beclin-1, and enhances cell proliferation, while sustained overexpression of XBP1s induces apoptosis, thus decreasing cell proliferation.65 In addition to apoptosis, many other pathways are involved in IRE1α-mediated atherosclerosis. Recently, Yildirim reported that IRE1 kinase activation induces fragile X mental retardation protein (FMRP) phosphorylation, and suppresses the macrophage cholesterol efflux and efferocytosis.101 IRE1α also plays a role in macrophage-derived foam cell formation by taking up more oxLDL, which is partly mediated by CD36.107 In addition, IRE1α regulates the IL-1β secretion in lipid-stimulated mouse BMDMs and human peripheral blood monocytes.100 In addition, XBP1s promotes macrophage-mediated inflammation through TNF-α and IL-8 production, and foam cell accumulation.108
In addition to its role in ECs and macrophages, the IRE1α-JNK pathway is also involved in oxLDL-induced apoptosis in VSMCs.109 In addition, XBP1 drives the plasma cell maturation and protective antibody responses.110 B-cell-specific XBP1 deficiency increases the apoptotic cell accumulation and antibody deposition in atherosclerotic plaques in Ldlr−/− mice and increases the necrotic core area.110
3.2 Systemic hypertension and pulmonary arterial hypertension (PAH)
Currently, the role of IRE1α in hypertension remains largely unknown. In animal models, the protein levels of IRE1α, p-IRE1α, and XBP1 are elevated in SHR arteries.111-113 The oxidation of IRE1α is partly regulated by nicotinamide adenine dinucleotide phosphate oxidase (Nox)-4, and may serve as a counterregulatory mechanism against oxidative stress in hypertension.111 Moreover, the administration of an IRE1α inhibitor reduces the proliferation of VSMCs in SHRs.111
PAH is a life-threatening disease characterized by increased pulmonary vascular resistance and vascular remodeling.114 In a rat model of PAH, the protein expression of IRE1α, p-IRE1α, and XBP1s was significantly greater in the lung tissues of rats with PAH than in those without PAH,115-119 and knocking down XBP1s ameliorated PAH in rats.115, 116 Vascular remodeling is largely due to the proliferation and resistance of pulmonary artery smooth muscle cells (PASMCs) to apoptosis.116 The upregulation of p-IRE1α and XBP1s is also observed in PASMCs in PAH.120 Recently, Jiang et al. reported that XBP1s promotes the proliferation, migration, and apoptotic resistance of PASMCs through the p-JNK/mitogen-activated protein kinase (MAPK) pathway.115 The overexpression of XBP1s also increases the expression of cell cycle-related proteins.120
3.3 Aortic aneurysm and aortic dissection
Aneurysm is a life-threatening vascular disease that is characterized by a permanent aortic dilatation.121 A study analyzing the expression of ER stress markers in abdominal aorta samples from abdominal aortic aneurysm (AAA) patients (n = 96) and healthy controls (n = 17) revealed that IRE1α/XBP1s levels are significantly greater in patients with AAA than in healthy controls, and the differences remained statistically significant after adjusting for age, sex, smoking status, hypertension status, and diabetes status.122 Moreover, strong immunostaining for XBP1 is observed mainly in VSMCs in the medial layer and, to a lesser extent, in lymphocytes in the inflammatory infiltration area of the aneurysmatic wall.122 In animal models, the expression of XBP1s is increased in AAAs in angiotensin II-treated ApoE−/− mice.123 Interestingly, Zhao et al. reported that XBP1u, not XBP1s, is repressed in the early stage of aneurysm formation in angiotensin II-infused ApoE−/− mice, which is correlated with VSMC dedifferentiation.121 XBP1u maintains VSMC homeostasis through FoxO4 interactions.121 In addition, XBP1u deficiency facilitates VSMC switching to a proinflammatory and proteolytic phenotype, and stimulates both thoracic and abdominal aortic aneurysm formation in mice.121 Recently, Chen et al. analyzed the functional enrichment of differentially expressed genes on the basis of Gene Expression Omnibus microarray datasets, and the results revealed that the expression of the IRE1α pathway is significantly upregulated in intracranial aneurysms and that the IRE1α pathway is highly related to FKBP14, Bax, and SEC61B expression.124
Aortic dissection is a rare but catastrophic disease with an extremely high mortality rate.125 The protein expression of p-IRE1α and XBP1s is significantly greater in aortic specimens from patients with aortic dissection than in those from patients without aortic dissection.125, 126 Moreover, the phosphorylation of IRE1α is also increased in animal models of aortic dissection and angiotensin II-treated rat aortic SMCs.126 Angiotensin II can promote the nuclear translocation of XBP1s, further resulting in the gene transcription of CHOP, cleaved caspase 3, Bax, and Bcl-2.125 In addition, Shi et al. revealed that the IRE1-XBP1-CHOP axis accelerates the apoptosis of VSMCs in the aortic media and promotes aortic dissection.126
3.4 IRE1α is involved in the pathological process related to vascular diseases
Neointimal hyperplasia is a universal response to vascular injury and is the leading cause of vascular restenosis.127 The known risk factors for neointimal hyperplasia include vascular injury, systemic inflammation, diabetes, and turbulent flow.128 p-IRE1α and XBP1s are increased in rat carotid artery balloon-injured, mouse wire-injured, and disturbed flow-induced neointimal hyperplasia models.129-131 In addition, the IRE1α/XBP1s axis is involved in increased endothelial-mesenchymal transition and subsequent carotid artery stenosis.98 The proliferation and migration of VSMCs play essential roles in the progression of neointimal hyperplasia. In vitro, both p-IRE1α and XBP1s are upregulated in the platelet-derived growth factor (PDGF)-BB stimulated human and rodent VSMCs.129-131 Inhibiting the IRE1α pathway reduces the hypertrophy and proliferation of VSMCs, which is partly mediated via inhibition of the NF-κB pathway.132 XBP1s increases the proliferation of VSMCs through the PI3K/Akt pathway and enhances the proliferation of VSMCs by suppressing calponin h1 and modulating the PDGF/transforming growth factor (TGF)-β pathway.131 Moreover, Angbohang et al. reported that XBP1s in VSMCs upregulates type IV collagen alpha 1/2 (COL4A1/2) transcription and induces soluble COL4A1 secretion, further recruiting stem cell antigen 1-positive-vascular progenitor cells (VPCs) and leading to neointimal formation.133
Vascular calcification is considered as a pathological process associated with CVD, including atherosclerosis, hypertension, and coronary artery disease.134 It has been well-documented that VSMCs play indispensable roles in the progression of vascular calcification.3 The phosphorylation of IRE1α and increased expression of XBP1s are observed in calcified VSMCs.46, 135 The expression of p-IRE1α and XBP1 is increased in bone morphogenetic protein-2 (BMP2)-induced human coronary artery smooth muscle cells (HCSMCs).136 XBP1s is also upregulated in stearate-stimulated VSMC osteoblastic differentiation and mineralization.137 XBP1s not only directly binds to the promoter of Runx2 in HCSMCs,136 but also promotes the activation of β-catenin/T-cell factor, the key regulator of vascular calcification, and facilitates Runx2 and Msx2 transcription.46 Overexpression of XBP1s accelerates high phosphate-induced VSMC calcification, and inhibition of XBP1s through IRE1α siRNA alleviates VSMC calcification29. In addition, OxLDL promotes osteoblastic differentiation through the IRE1α/XBP1s/Runx2 pathway and activates inflammation through the IRE1α/JNK and NF-κB pathways.138 The IRE1α/JNK axis is also activated in parathyroid hormone-induced HASMCs.139 Recently, Yang et al. reported that XBP1u protects against calcification via an ER stress-independent signaling pathway.46 The XBP1u level is lower in the radial arteries of chronic kidney disease patients with calcification than in those without calcification.46 Smooth muscle-specific knockout of XBP1u aggravated 5/6 nephrectomy and adenine diet-induced vascular calcification.46 Interactome analysis revealed that XBP1u binds directly to β-catenin, promotes its ubiquitin-proteasomal degradation, and inhibits Runx2 and Msx2 transcription.46
3.5 IRE1α is involved in vascular diseases mediated by common risk factors
Age is considered as an important risk factor for vascular diseases. Bioinformatic analysis of transcriptome datasets revealed that the expression of XBP1 is upregulated in aged ApoE−/− mice.140 The phosphorylation of IRE1α is also observed in the aortas of aged mice and aged ApoE−/− mice.140, 141 In addition, the protein levels of p-IRE1α and XBP1s are increased in aged HUVECs.141, 142 Compared with that in young fibroblasts, the IRE1α/XBP1s axis is also activated in aged fibroblasts.143 In aging cells, activated Nox4 is involved in the dissociation of heat shock protein 90 from IRE1α.142 Additionally, IRE1α-mediated RIDD facilitates premature senescence through the degradation of Id1 mRNA.144
Smoking is a leading cause of CVD. Smoking increases the risk of mostly CVD subtypes, and the risk increases with smoking intensity.145 Cigarette smoke extracts significantly activate the IRE1α/XBP1s axis.146, 147 The phosphorylation of IRE1α is also induced in nicotine-induced human coronary artery ECs.148
Previous studies have demonstrated that increased levels of circulating advanced glycation end products (AGEs) promote vascular diseases.149-151 IRE1α/JNK have been reported to participate in AGE-induced cell apoptosis.152, 153 AGEs induce the phosphorylation of IRE1α and the JNK-signaling pathway in a time- and dose-dependent manner in HUVECs.154 AGEs also directly induce XBP1 expression in HAECs and play a role in apoptosis, and further lead to endothelial dysfunction.149 IRE1α knockdown inhibits AGE-induced NF-κB p65 nuclear translocation and the inflammatory response.154, 155 In addition, IRE1α knockdown suppresses Bax upregulation, Bcl-2 downregulation, and caspase-3 activation, thereby ameliorating apoptosis.153
4 TARGETING IRE1α IN VASCULAR DISEASES
Given the role of IRE1α in the pathogenesis of vascular diseases, strategies targeting IRE1α are emerging as potential therapeutic avenues for disease intervention. Two IRE1α RNase specific inhibitors, STF-083010 and 4μ8c, are administered in the models of atherosclerosis.100 STF-083010 is an inhibitor of IRE1α RNase activity that does not affect its kinase activity and selectively inhibits XBP1 mRNA splicing.156 4μ8c is also a small molecule inhibitor that selectively inhibits RNase activity of IRE1α. Compared with STF-083010, 4μ8c also inhibits RIDD mRNA degradation.157 Both STF-083010 and 4μ8c suppress lipid-induced mitochondrial reactive oxygen species (ROS) production and inflammasome activation in macrophages and block the Th-1 immune responses and IL-18 cytokine release, which further reduce the plaque area and alter the plaque composition of ApoE−/− mice .100 In addition, the IRE1α kinase-specific inhibitor, AMG-18, prevented the phosphorylation of IRE1α, reduced the phosphorylation of FMRP, and significantly reduced the foam cell area and necrotic core area of atherosclerotic lesions in ApoE−/− mice.101 AMG-18 also increases the efferocytosis of apoptotic cells in both peritoneal macrophages and BMDMs.101
In addition to the roles of the STF-083010 and 4μ8c in atherosclerosis, their roles have also been reported in other vascular diseases. For example, 4μ8c has been reported to prevent pulmonary vascular remodeling and PAH through inhibiting the IRE1α/XBP1s pathway.120 The administration of 4μ8c also promotes apoptosis and inhibits the proliferation and migration of PASMCs.118 Moreover, 4μ8c decreases the senescence-associated β-galactosidase production in fibroblasts.143 In SHRs, using the compound STF-083010 disrupts the IRE1α/XBP1s pathway and reduces the proliferation of VSMCs.111 In addition, STF-083010 can block the production of proinflammatory factors and the expression of adhesion molecules in HUVECs.158 STF-083010 also reduce the degree of endothelial-mesenchymal transition in primary mouse aortic ECs exposed to disturbed flow.98 MKC-3946, an IRE1α RNase domain inhibitor, was found to reverse the XBP1s-associated ROS production, phenotype switch, and apoptosis of VSMCs, further alleviating aortic dissection.125 These findings above underscore the therapeutic potential of IRE1α inhibitors in the management of vascular diseases.
5 CHALLENGES FOR FUTURE RESEARCH
Accumulating evidence indicates that IRE1α plays an important role in the pathogenesis of vascular disease. In this context, there are many attempts to manage vascular disease by administering IRE1α inhibitors. Pharmacological modulation of IRE1α activity through targeting either the RNase domain or the kinase domain has shown potential for the management of vascular diseases. However, most of these studies were conducted ex vivo or in vitro, which could neither mimic the comprehensive environment in humans nor accurately represent the pathological changes in human disease. Therefore, clinical studies are warranted to validate the results of these studies. Clinical trials assessing these inhibitors are still lacking, and it remains to be discovered how these strategies can be applied to the clinical studies. To translate the current knowledge about the IRE1α-signaling pathway into clinical treatment for vascular disease is still challenging. In addition, globally targeting IRE1α may induce severe adverse effects. Thus, selectively targeting IRE1α in an organ-specific or organelle-specific manner might be a promising way in the treatment of vascular diseases.
6 CONCLUSIONS
Emerging evidence suggests that the IRE1α-signaling pathway contributes to the progression of vascular diseases, including atherosclerosis, systemic hypertension and PAH, aortic aneurysm, and aortic dissection, by mediating apoptosis and autophagy, and trigger inflammatory response. Currently, most studies on the role of IRE1α in vascular diseases originate from animal studies and in vitro studies, which largely limits our understanding of the pathological changes in human diseases. In addition, although small inhibitors targeting IRE1α have potential roles in the prevention and treatment of vascular diseases, clinical trials are still lacking. Therefore, further investigations are expected to identify the precise role of IRE1α and develop new therapeutic strategies.
AUTHOR CONTRIBUTIONS
JS wrote the main manuscript text and prepared figures. FH and XGD revised the final version. All the authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (Grant number: 82300832), the Natural Science Foundation of Chongqing Science and Technology Commission of China (Grant number: cstc2019jcyj-msxmX0504), and the research projects of Yuzhong district of Chongqing (Grant number: 20190105).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ETHIC STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




