Management of a Borderline Brenner Tumor of the Ovary in a Premenopausal Woman in Northern Tanzania. A Rare Case Report and Review of the Current Literature
Funding: The authors received no specific funding for this work.
John Lugata and Laetitia Makower are co-first main authors and contributed equally to the drafting of this manuscript.
ABSTRACT
Brenner tumors are rare ovarian neoplasms that are typically small and unilateral. Most cases are benign; less than 5% of all Brenner tumors are borderline or proliferative. Brenner tumors originate from the follicular epithelium and consist of ovarian transitional cells surrounded by fibrous tissue. Here we present a case of a 49-year-old premenopausal woman from Northern Tanzania. This patient presented to our facility with acute abdominal pain. She also reported abdominal distention which was first noticed 12 months prior to presentation. Magnetic resonance imaging (MRI) identified a 21 × 17 × 5 cm complex cyst with a thickened wall on the right ovary. The radiological findings were suggestive of an ovarian cystadenoma; multiple intramural and subserosal uterine leiomyomas were also noted. A total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) was performed. Histopathological findings identified an atypical proliferative borderline Brenner tumor of the right ovary. Postoperative recovery was uneventful, the patient was discharged and managed conservatively postoperatively.
Summary
- Borderline Brenner tumors are rare and pose diagnostic challenges due to their non-specific radiological and serological features.
- Clinicians should maintain a high index of suspicion when encountering atypical ovarian masses, especially in premenopausal women. Surgical management remains the primary treatment, with a good prognosis when complete resection is achieved.
- This case highlights the importance of accurate histopathological assessment to guide appropriate treatment. Regular follow-up is crucial to monitor for recurrence, although the risk remains low with effective surgical intervention.
1 Introduction
Brenner tumors are a unique histologic subtype of epithelial ovarian tumors; they comprise less than 3% of all ovarian neoplasms. These tumors are named after Dr. Fritz Brenner who initially described three cases in 1907. He labeled them “oophoroma folliculare” believing that the tumor nests may have been abnormally developed ovarian follicles [1]. Brenner tumors are typically unilateral and are distinguished from other ovarian neoplasms by their unique epithelial lining. The epithelial lining of Brenner tumors contains clusters of transitional cells resembling the urinary tract epithelium. Occasionally, these nests feature microcysts or mucinous glands at their center [2]. The World Health Organization (WHO) has categorized Brenner tumors into three subtypes based on histopathological features and clinical prognosis; cases are either malignant, borderline, or benign. Benign Brenner tumors are the most common, borderline and malignant Brenner tumors are rare accounting for roughly 95%, 5%, and 1% of all cases, respectively [3]. Brenner tumors are usually asymptomatic and are often found incidentally; however, larger tumors may present as a pelvic mass accompanied by severe pain [4]. Brenner tumors can develop in women of any age but are most common in postmenopausal women between the fifth to seventh decade of life [5]. The preferred treatment for borderline Brenner tumors is surgical resection, as no additional therapy is currently recommended. It is noteworthy that borderline Brenner tumors have a favorable prognosis [6]. In this study, the authors report a rare case of a borderline ovarian Brenner tumor in a premenopausal woman in Northern Tanzania, who was managed conservatively after surgery.
2 Case History
A 49-year-old premenopausal woman (G2P2) presented to our emergency department complaining of acute abdominal pain and mild nausea which had been present for 1 day. The pain was localized to the right lower quadrant, it was dull and of gradual onset. This acute presentation was preceded by a 1-year history of abdominal distension, anorexia, and early satiety. The patient denied any per vaginal bleeding or discharge. Past gynecological history was unremarkable, including a regular menstrual cycle and no menorrhagia. However, the patient had been experiencing irregular menstrual bleeding for 6 months indicating a perimenopausal state. Drug history included the combined oral contraceptive pill and regular medication for hypertension and peptic ulcer disease. There was no relevant personal or family history for ovarian, uterine, bowel, or breast cancer.
On examination, the patient was well perfused, breathing comfortably at rest and afebrile. The patient was hypertensive with a reading of 160/83 mmHg, all other observations including blood glucose were within normal range. Examination of the abdomen revealed marked distention, rigidity, and tenderness in the right lower quadrant. Palpation identified a smooth firm mass in the pelvis and no hepatosplenomegaly. Bowel sound were not clearly audible and lower limb pitting edema was not present. On vaginal examination, there were no visible abnormalities of the cervix, it was noted both vaginal fornices were full. The examination of other systems was unremarkable.
3 Methods
Following the patient's initial presentation the leading differential diagnoses included serous cystadenoma, retroperitoneal tumors, and mucinous cystadenocarcinoma. Cancer antigen 125 (CA-125) and carcinoembryonic antigen (CEA) tumor markers were markedly elevated. βHCG and α-fetoprotein were within normal limits. Further blood tests were undertaken including a complete blood count, liver and renal function tests, all were within normal limits. Cardiac investigations including an ECG and ECHO were also normal. A pelvic MRI identified a well-defined septated cystic lesion arising from the right adnexa. The mass measured 15 × 26 × 27 cm, no enhancing solid components were present. These features are consistent with a right ovarian cystadenoma. Furthermore, the uterus appeared bulky with multiple intramural and subserosal uterine leiomyomas, the largest of which measured 7 × 7 × 7 cm and arose from posterior wall of the uterus.
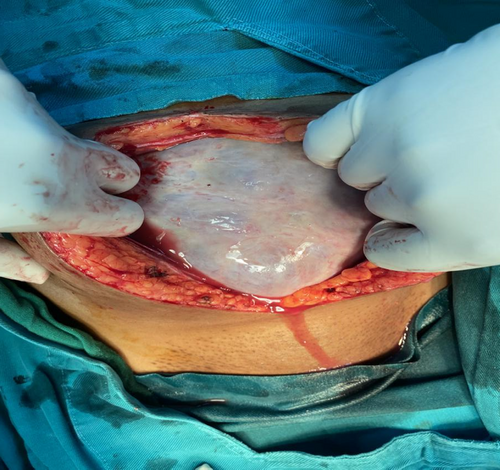
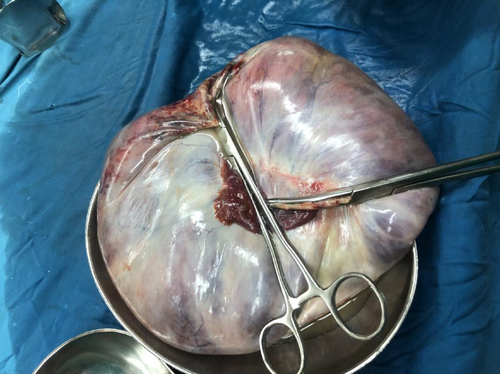
The patient was prepared for an explorative laparotomy under general anesthesia, with a left lateral tilt to the operating table. The abdomen was opened in layers with an extended umbilical midline incision. A large cystic mass was identified on the right ovary measuring 20 × 20 × 19 cm (Figure 1). There was no obvious solid component to the mass and minimal ascitic fluid was present. Additionally, the uterus appeared bulky with multiple subserol and intramural uterine fibroids. A TAH and BSO was performed and hemostasis was achieved. The specimen was submitted for histopathological evaluation (Figure 2).
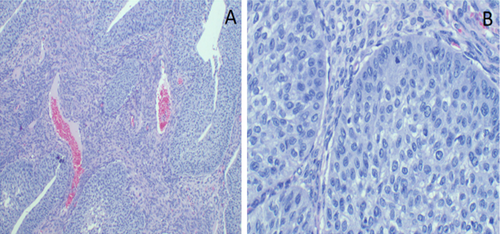
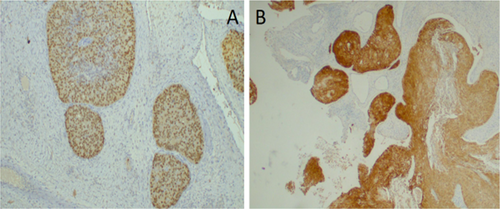
The postoperative recovery was uneventful. Histopathological analysis identified a borderline Brenner tumor composed of atypical transitional Brenner tumor cells developing from an area of benign transitional cells. Nest of cells were clearly identified without stromal invasion (Figure 3A,B). Further analysis with immunohistochemistry was performed, the cells stained positively for p63 and GATA3 (Figure 4A,B), but were negative for estrogen and progesterone receptors.
4 Conclusion and Results
Postoperative care was provided on the general ward. Intravenous antibiotics and analgesia were administered. The patient was discharged on the sixth postoperative day and returned to the gynecology clinic for follow-up 8 days later. Regular follow-up was undertaken and the patient was seen every 4 months for 2 years in order to monitor her progress. To date, regular follow up has shown no evidence of tumor recurrence, with negative radiologic finding from transvaginal ultrasounds and chest, abdomen, and pelvis computed tomography scans. Furthermore, the elevated serum CA-125 and CEA levels resolved following surgery.
5 Discussion
Our report describes a case of a large borderline Brenner tumor in a premenopausal woman. These tumors are typically asymptomatic and identified incidentally [7]. Literature on this topic is scarce as Brenner tumors account for only 3% of ovarian cancers; borderline cases are rarer still representing only 5% of all Brenner tumors [8].
Histologically, borderline Brenner tumors resemble low-grade papillary urothelial neoplasms. Both these neoplasms feature uniform cells, elongated nuclei, fine chromatin, and prominent nucleoli. However, some cases also exhibit mucinous or squamous metaplasia, as well as moderate to severe atypia [9]. Brenner tumors consist of nests of transitional/urothelial-type epithelium, which are surrounded by a dense fibromatous stroma [10]. They are thought to originate from the ovarian surface epithelium through transitional-type metaplasia. However, recent theories suggest that Brenner tumors may in fact originate from the fallopian tube epithelium, undergo transitional cell metaplasia, and then spread to the ovaries [10]. Current literature describes several rare cases of Brenner tumors; these tumors have been associated with recurrent urothelial carcinoma of the urinary bladder [11] and HPV-associated squamous cell carcinoma [12].
The most common clinical manifestations of borderline Brenner tumors are abdominal enlargement or a mass, abdominal pain, and postmenopausal bleeding [6]. The patient is our cases presented with nausea and acute on chronic unilateral lower abdominal pain. The patient also reported symptoms relating to the presence of a large mass in her abdomen including bloating, early satiety, and anorexia. Vital signs were largely normal excluding mild hypertension. Abdominal examination identified distension, a palpable mass and a rigid and tender abdomen. The patient was premenopausal at diagnosis. Borderline Brenner tumors can occur in patients between the ages of 30–84, but are more prevalent in elderly patients, with over 80% of patients being older than 50 at diagnosis [6, 13].
The literature does not illustrate a convincing serological picture of a Brenner tumor. For example, there is no direct correlation between CA-125 levels and borderline tumors [6]. CEA and CA-125 levels were elevated in our patient, yet Brenner tumors have not been associated with a reliable tumor marker [14]. Borderline Brenner tumors stain positively for Ras, EGFR, CK5/6, CK7, P63, CA-125, epithelial membrane antigen, GATA3, and S100P, and stain negatively for p16, Rb, p53, CK20, and CDX2 [10, 15].
As was seen in our patient, borderline Brenner tumors are composed of transitional-type epithelium which resembles a low-grade non-invasive papillary transitional cell neoplasm of the urinary tract [3]. Similarly, nearly all cases display areas of benign transitional cells; the proliferating component arise directly from these areas, as it was noted in our patient. The cytological features are similar to those found in benign transitional cell tumors, but occasionally significant atypia and mitotic activity are present. Some authors have separately classified “proliferative” and “low malignant potential” transitional cell tumors. Stromal invasion by irregular nests of cells or single cells in an infiltrative pattern are malignant features, none were present in our case.
It is challenging to diagnose Brenner tumors by radiological studies, as there are no specific pathognomonic features of these tumors [16]. Additionally, the imaging characteristics distinguishing benign from malignant Brenner tumors have been inadequately described [14]. In our case a pelvic MRI was used as the primary imaging tool and identified a well-defined septated cystic lesion in the right adnexa. There was no evidence of solid components within the mass. Overall, the imaging supported a diagnosis of an ovarian cystadenoma. Herein highlights the challenge clinicians face when attempting to diagnose Brenner tumors pre-operatively, the imaging findings are non-specific and therefore support more common diagnoses such as cystadenomas. Furthermore, borderline and malignant cases share radiological features such as papillary structures and cystic regions [17]. Hence it is difficult to develop and study different treatment approaches for borderline and malignant cases. The ability to differentiate between borderline and malignant cases pre-operatively plays a vital role in determining the most appropriate therapeutic strategy. By employing advanced imaging and molecular diagnostic test preoperatively, surgeons can better distinguish between benign and malignant ovarian masses, thereby minimizing unnecessary radical surgeries in cases where less invasive procedures may be appropriate. This is crucial as unnecessary surgical morbidity can be avoided in patients with benign masses, and minimally invasive surgery (MIS) has been shown to lead to fewer postoperative complications and quicker recovery times compared to open surgeries [18, 19].
Literature on the topic of Brenner tumors is limited, it is therefore interesting to consider the current treatment regimes. As demonstrated by our case the current treatment for borderline Brenner tumors is surgical resection [6]. The staging used for borderline Brenner tumors is identical for that of malignant Brenner tumors [6], the aggressive nature of treatment likely reflects this. When comparing “no evidence of disease” periods, there was no discernible difference between a TAH and BSO, as used in this case, and a unilateral salpingoophrectomy [6].
In order to develop treatment regimes that consider borderline tumors as their own entities, rather than analogous to malignant cases, the literature should discuss outcomes of various approaches to borderline tumors. Furthermore, as our case describes a borderline Brenner tumor in a premenopausal patient, we must consider the possibility of a fertility sparing approach. A single case report in the literature has demonstrated the feasibility and success of unilateral salpingoophrectomy as a fertility sparing approach whereby the patient successfully spontaneously conceived following surgery [7].
Our case highlights the importance of contributing case reports to the literature in order to compare current treatment options and inform patient centered management. The prognosis of borderline Brenner tumor is good in general. In the cases we reviewed, only one lethal recurrence was reported. The patient had two recurrences and died from the last recurrence without histologic confirmation [8].
Future research in the field may consider the use of radiation therapy (RT) as treatment for Brenner tumors. Due to the unfavorable side effect profile of whole abdominal radiation therapy (WART) and the tumors' relative responsiveness to chemotherapy, RT has become less popular for treating epithelial ovarian cancers [20]. NCCN guidelines discuss the use of palliative RT for symptom control of epithelial ovarian malignancies such as Brenner tumors [21]. Given the rarity of Brenner tumors the use of adjuvant chemoradiation therapy has not been thoroughly investigated or standardized. However, it has been shown that adjuvant platinum–taxane treatment produced a favorable outcome after surgical excision of malignant Brenner tumors [22]. Patients with local recurrence may also benefit from targeted radiotherapy following surgical resection. More research is required to develop further guidelines regarding the use of adjuvant therapies.
In conclusion, the case presented here illustrates a rare case of a large unilateral Brenner tumor in a premenopausal woman. This case highlights the importance of adding reports to the current literature base in order to develop treatment protocols which are specific to borderline tumors. The challenges presented by non-specific radiological features makes pre-operative diagnoses of Brenner tumors difficult. In clinical practice, detecting tumors at an early stage will facilitate effective treatment and management strategies.
Author Contributions
John Lugata: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing – original draft, writing – review and editing. Laetitia Makower: conceptualization, formal analysis, investigation, methodology, validation, writing – original draft, writing – review and editing. Ashley Rapheal: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing. Yusuph Mwidibo: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing. Bariki Mchome: conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, writing – original draft, writing – review and editing. Alex Mremi: conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, writing – original draft.
Acknowledgments
The authors would like to thank the patient for the permission to use her medical information to be shared for learning purposes in this publication. We equally appreciated the contributions, support and input from Highness Lawuo, Doreen Mwandike, Epifania Venance, and Asiya Ali Khalfan in this work.
Ethics Statement
The patient provided written informed consent to allow for her de-identified medical information to be used in this publication. A waiver for ethical approval was obtained from the authors' institution review board committee.
Consent
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
There is no data generated from this study.




