Gastrointestinal stromal tumor (GIST) mistaken for pancreatic pseudocyst – case report and literature review
Key Clinical Message
A 74-year-old female patient underwent a Roux-en-Y cystjejunostomy for pancreatic pseudocyst developed several melena episodes and she was surgically reappraised. The main diagnostic concern was a pancreatic cystic neoplasm. A 12 × 8.0 × 5.0 cm retro-gastric lesion was resected and pathology report indicated an unsuspected gastrointestinal stromal tumor (GIST). The report aimed to describe an atypical presentation of GIST.
Introduction
Medical literature has previously shown that a pancreatic cystic neoplasm could be mistaken for a pancreatic pseudocyst. Cystic tumors of the pancreas most commonly corresponds to serous microcystic adenoma, intraductal mucinous tumor, mucinous cystic tumor, and solid pseudopapillary tumor and, less commonly to cystic endocrine tumors, cystic metastasis, cystic teratomas, and lymphagiomas 1. The case reported aimed to redefine the differential diagnosis of a presumed pancreatic pseudocyst, emphasizing the possibility of GIST.
Case Report
A 74-year-old female patient was admitted to the internal medicine ward of São Paulo Hospital in April, 2012. The main complaint was gastrointestinal bleeding identified as melena, occurring some weeks after a Roux-en-Y cystjejunostomy for a pancreatic pseudocyst. Bleeding was not, initially, thought to be related to the previously done cystjejunostomy, as it is an allegedly uncommon complication of that procedure. Investigation was carried out with upper gastrointestinal endoscopy (UGE). Several gastric erosions were observed but thought to be unrelated to the patient′s bleeding, and the urease test was positive for Helicobacter pylori (HP) infection. Although there was no evidence of gastric bleeding, the initial approach consisted of a proton pump inhibitor and antibiotic therapy, for HP.
Because the melena persisted, the patient underwent colonoscopy, which revealed diverticulosis in both the ascending and transverse colon. There was no evidence of recent bleeding. Other diagnostic procedures included an abdominal CT scan and Technetium-99 m-labeled RBC scintigraphy, both of which had nonspecific findings and were not diagnostic. Repeat UGE showed complete resolution of the gastric erosions which led to the search for a lesion in the small bowel. By this point in her clinical course, the patient had required 20 packed red cell transfusions.
A small bowel barium study revealed findings compatible with a retro-gastric tumor causing extrinsic compression of the stomach.
At that time, the medical team considered that the main diagnostic concern was a pancreatic cystic neoplasm masquerading as a pancreatic pseudocyst and the patient underwent a laparotomy (Fig. 1).
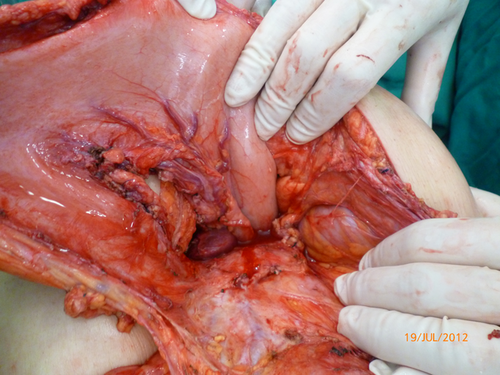
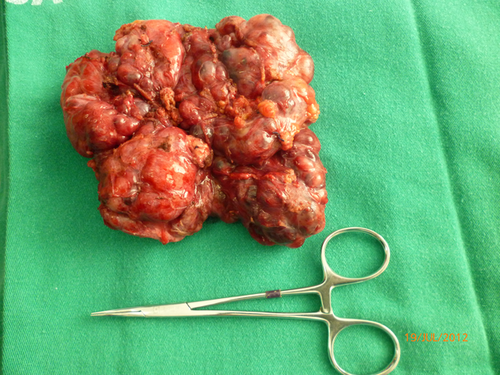
At surgery, a retro-gastric lesion measuring 12 × 8.0 × 5.0 cm was resected and the pathology report indicated an unsuspected gastrointestinal stromal tumor (GIST) (Fig. 2).
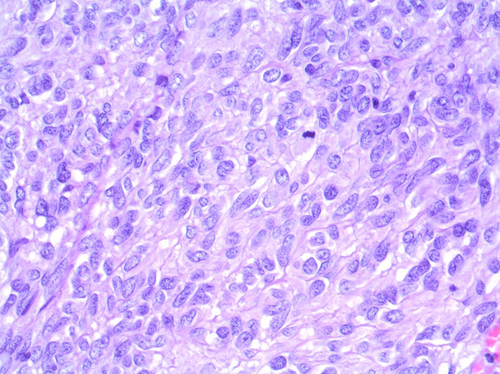
Histologic findings were an epithelioid and fusiform mixed pattern tumor, with high mitotic grade (21 of 50 high-power fields) and central cystic degeneration. By immunohistochemistry, the tumor expressed C-KIT, vimentin, DOG 1, and KI 67 (Figs. 3 and 4).

Discussion
GISTs are rare, with an estimated incidence of 1.5/100.000 per year 2 and encompass tumors primarily derived from Cajal interstitial cells – pacemaker cells which regulate gastrointestinal tract motility 3. By immunohistochemistry, such tumors are usually tyrosine kinase receptor “KIT” positive. In fact, the KIT mutation leads to kinase activation, and experimental studies have demonstrated that the mutation is capable of causing Cajal cells hyperplasia and GIST-like tumors in mice 3.
Most GISTs appear on the fifth to seventh decades of life. Any part of the gastrointestinal tract – from esophagus to rectum – may be involved, although stomach (60%) and small bowel (30%) are the predominant organs affected. The clinical expression of disease tends to be with gastrointestinal bleeding and nonspecific abdominal complaints. Some tumors from outside the gastrointestinal tract are called extragastrointestinal stromal tumors or EGISTs; most of them are from the omentum, mesentery or retroperitoneum 3.
GIST pathology report encompasses three tumor subtypes: a fusiform cell (70%), an epithelioid cell (20%), and a mixed pattern. The fusiform cell subtype correlates with the KIT mutation (CD117 positivity), the tumor cells present a spindled phenotype, and the clinical course of such tumors is usually less aggressive. The other histological patterns (epithelioid cells and mixed pattern) correlate with platelet-derived growth factor receptor alpha (PDGFRA) expression (CD34 positivity), although such correlation does not consistently occur.
Tumors with a predominantly epithelioid pattern and PDGFRA mutation tend to have a favorable prognosis. On the other hand, an epithelioid or mixed pattern accompanied by the KIT mutation – not PDGFRA mutation – which occurs particularly in GISTs of the stomach, tends to have an unfavorable course.
By immunohistochemistry, 95% of GISTs express CD117, and these KIT mutated tumors respond to therapy with KIT inhibitors (imatinib or sunitinib). Less than 5% of GISTs are KIT immune-negative; many of them have the PDGFRA mutation and respond to targeted therapy as well. A third type, called “wild” type and without either KIT or PDGFRA mutation, has been described too. It tends to be therapy-resistant.
Other histological markers are S-100 protein (5%), desmin (2%), a recently described “discovered on GIST” protein (DOG1, 95%) 3 and Ki-67, the expression of which correlates with a high mitotic grade of tumor cells and with a worse prognosis 4. Vimentin is almost always positive.
In the reported case, by the time the patient had the second operation, the medical staff had already estimated gross probabilities of different diagnostic possibilities.
First, bleeding should be regarded as a potential surgical procedure complication. According to Newell et al. (1990), bleeding after Roux-en-Y cystjejunostomy is definitely an uncommon complication, occurring in only 2% of the cases 5.
Second, a colonic source of bleeding should be considered despite a presentation as melena. Previous studies have focused on the diagnostic yield of colonoscopy in the investigation of bleeding after a negative UGE; positivity rates of 23.5% to 30% were reported. The main limitations observed were overvaluing the role played by lesions with low implication for bleeding and small sample sizes 6, 7. A recently published study with better design has reported a 14.2% positivity rate for colonoscopy (4.8% if only lesions with a high implication for bleeding are included) 8.
Finally, melena should be considered to result from some previously neglected condition, for example, a pancreatic cystic neoplasm mistaken for a pancreatic pseudocyst 9. Previous reports estimate that this occurs in 9–12% of the cases 10.
Other possibilities would be small bowel carcinoid 11, Dieulafoy′s lesion 12 and other rare diseases.
Combining data from those reports, it could be estimated a 2% bleeding probability related to the cystjejunostomy previously carried out; a 4.8% melena probability from a colonic source; finally, a 12% bleeding probability from an unsuspected pancreatic cystic neoplasm. The upper gastrointestinal bleeding investigation points to the small bowel whenever UGE and colonoscopy are unrevealing, and endoscopic capsule and small bowel study should be considered. In the case presented here, a small bowel barium examination was able to uncover a suspicious lesion.
By means of probabilistic reasoning, a pancreatic cystic neoplasm was the pathologic condition to be ruled out.
To our knowledge, GIST masquerading as pancreatic pseudocyst is rather unusual, and we found only one prior report of such an atypical presentation in the medical literature 13. In fact, the case reported by Aggarwal and colleagues was a metastatic gastric GIST mistaken for pancreatic pseudocyst. On the other hand, the case reported here seems to be a primary retroperitoneal GIST. This type of tumor, actually known as EGIST, seems to be a rare medical finding.
A case series from the National Cancer Institute of Brazil (INCA) reported only nine EGIST cases over a period of 13 years from 1997 to 2009 14. Primary EGIST of the retroperitoneum seems even rarer, with only 58 cases reported in the medical literature according to Casella and colleagues 15.
In conclusion, it is not an overstatement to say that any presumed pancreatic pseudocyst merits double attention. It might be a pancreatic cystic neoplasm – as the medical literature has previously shown – or even a GIST, as this report has emphasized.
Acknowledgment
The authors would like to acknowledge the invaluable help and advice from Dr Steven Weinberger.
Conflict of Interest
None declared.




