Co-occurrence of rhino-orbital mucormycosis and acute lymphoblastic leukemia post-COVID-19 infection in a young adolescent male: A case report from a low middle-income country
Abstract
Key Clinical Message
Immunosuppression from B-acute lymphoblastic leukemia (B-ALL) chemotherapy and a preceding COVID-19 infection may predispose patients to rare complications such as rhino-orbital mucormycosis. Hence, a high index of suspicion should be maintained by physicians (and oncologists) if patients undergoing B-ALL treatment present with orofacial symptoms and ophthalmological manifestations such as peri-orbital swelling, ophthalmoplegia, and loss of vision, suggestive of infection.
Mucormycosis is a severe fungal infection that poses significant mortality and morbidity risks, particularly in immunocompromised individuals. We present a rare case of a 16-year-old patient with rhino-orbital mucormycosis following B-acute lymphoblastic leukemia (B-ALL) treatment and concurrent COVID-19 infection. We describe the clinical presentation, diagnosis, treatment, and outcome of this patient, and discuss the possible interactions and implications of these three conditions. A young 16-year-old male patient without significant clinical history was admitted with complaints of low-grade intermittent fever, fatigue, malaise, restlessness, and unexplained weight loss for the past 2 months. A bone marrow biopsy confirmed the diagnosis of B-ALL. Following the diagnosis of B-ALL, the patient underwent initiation of chemotherapy. Following the initial two cycles of chemotherapy, the patient experienced fever and cough and tested positive for COVID-19 infection. Nearly a week later, the patient presented to the chemotherapy emergency department with a clinical picture characterized by a fever up to 39°C associated with left facial swelling, severe headache, purulent rhinorrhea, and foreign body sensation in the ipsilateral nostril. The following day, erythema and left eyelid edema were observed, with ocular opening limitation. The diagnosis was confirmed based on the positive result of polymerase chain reaction for left-sided mucormycosis. Initial administration of liposomal and lipid amphotericin B at 1–1.5 mg/kg/d doses for 4–6 weeks was followed by surgical debridement of necrotic tissue on the left side of the face and nose. Subsequent ophthalmological examinations showed normal conditions of the left eye. The case underscores the importance of heightened clinical suspicion, early diagnosis through imaging and molecular techniques, aggressive multimodal therapy, and close interdisciplinary collaboration for improved outcomes in such rare and challenging clinical scenarios.
1 INTRODUCTION
Mucormycosis is one of the most prevalent fungal infections caused by mucoraceae found throughout the environment.1 The most common causative agents are fungi from the order Mucorales including Rhizopus, Rhizomucor, Lichtheimia, and Mucor.1 It has significant mortality and morbidity rates and acts as an invasive pathogen in immunocompromised patients (patients with hematological malignancies, transplant recipients, and people with uncontrolled diabetes).2 The clinical presentation is influenced by the involvement of the particular organ system it affects and varies based on its origin. It is classified into six clinical forms: rhinoorbito-cerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and infrequent rare forms (e.g., endocarditis and osteomyelitis).2 According to the SEIFEM study, mucormycosis is present in 0.1% of patients with hematological malignancies.3 Although patients with acute myeloid leukemia are more susceptible to invasive fungal disease than patients with acute lymphoblastic leukemia (ALL), a previous study reported that patients with ALL have a high risk of acquiring an invasive fungal disease despite the rapid development of antifungal medications in recent years.4 Despite early diagnosis and aggressive treatment, the disease carries a high mortality. Individualized multidisciplinary approaches are therefore recommended. This article aimed to present a rare case of 16-year-old patient with rhino-orbital mucormycosis after B-cell acute lymphoblastic leukemia (B-ALL) and concurrent COVID-19 infection following induction chemotherapy. This case report has been reported in line with the SCARE criteria.5
2 CASE PRESENTATION
2.1 Initial case history/examination
A young 16-year-old male patient without significant clinical history was admitted with complaints of low-grade intermittent fever, fatigue, malaise, restlessness, and unexplained weight loss for the past 2 months. The fever ranged between 99.5 °F (37.5°C) and 102.2 °F (39°C), the patient had been managing fever with regular paracetamol use, which temporarily alleviated symptoms for a few hours. While the vitals were stable, on physical assessment, the patient appeared pale with visible signs of weight loss. Respiratory, cardiovascular, abdominal, head and neck, and neurological examinations were normal.
2.2 Differential diagnosis and investigation
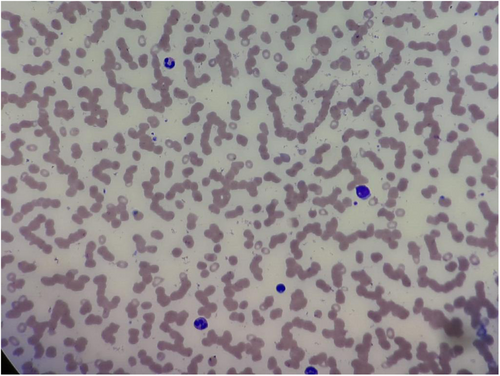
Rhinosinusal aspergilloma, Wegner granulomatosis, and pseudomonas lesions were among the differential diagnoses. The initial step in the diagnostic workup involved a complete blood count (CBC). Laboratory investigation of blood revealed normocytic anemia (Hb-7.8 g%, MCV-86f), white blood cell count of 4330 cells/mm3, and platelet count of 15,000/mm3. A peripheral smear showed severe thrombocytopenia, normocytic and microcytic RBCs, and 10% atypical mononuclear WBCs along with some immature myeloid precursors. Following the blood investigations, a bone marrow biopsy was performed to confirm the diagnosis as shown in Figure 1. It revealed a leucoerythroblastic picture with bicytopenia and 10% typical cells, thereby confirming the diagnosis of B-ALL. The blasts were found positive for CD10, CD19, CD20, CD34, CD38, CD58, HLA-DR, CD36, CD22, and TDT. However, no chromosomal abnormality was detected.
2.3 Treatment of B-ALL
A decision to start chemotherapy was made with the first phase of induction chemotherapy including the BFM-95 chemotherapy regimen consisting of vincristine, pirarubicin, prednisone, and pegaspargase. It was followed by the second phase of induction consisting of cyclophosphamide, cytarabine, and mercaptopurine. Consequently, protocol M (including methotrexate and mercaptopurine) was administered. Maintenance therapy included 6-mercaptopurine and methotrexate for 2 years, followed by four additional doses of high-dose methotrexate. Maintainance therapy was followed by an induction phase (consisting of vincristine, pirarubicin, prednisone, and pegaspargase). It was followed by a re-induction phase, which included cyclophosphamide, cytarabine, and mercaptopurine. Additionally, the patient was administered intrathecal injections of methotrexate, cytarabine, and dexamethasone.
2.4 Concurrent infection with COVID-19 and rhino-orbital mucormycosis
Following the initial two cycles of chemotherapy, the patient experienced fever and cough and tested positive for COVID-19 infection. His treatment was held temporarily for 5 days. None of the family members of the patient were reported to be infected with COVID-19. Later, the chemotherapy regimen was continued. However, 4 days later, the patient attended the chemotherapy emergency department presenting a clinical picture characterized by a fever up to 39°C associated with left facial swelling, severe headache, purulent rhinorrhea, and foreign body sensation in the ipsilateral nostril. The following day, erythema and left eyelid edema were observed, with ocular opening limitation and the presence of necrotic crusts in both nostrils. The patient was evaluated by the ophthalmology and otorhinolaryngology services, who considered a possible diagnosis of bacterial or fungal infection, so antimicrobial treatment with fluconazole and cefepime at therapeutic doses was started, showing unsuccessful response with sudden deterioration of the clinical picture, requiring transfer and management in the intensive care unit, and initiation of inotropic and vasopressor support measures.
2.5 Diagnostic imaging investigations
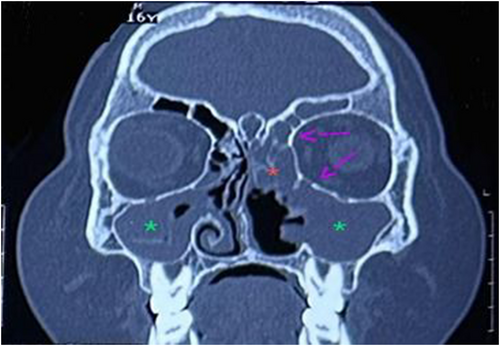
Once stable, an MRI was performed, which revealed inflammatory findings in the orbit and sinuses, with a widespread thickening of the mucosa in both the maxillary and sphenoid sinuses, suggesting inflammation (Figure 2). The left frontal sinus along with the left lower orbit showed mild mucosal thickening and inflammation. A post-contrast study showed nodular enhancement along the medial and inferior walls of the left orbit (Figure 3) consistent with a diagnosis of mucormycosis. In addition, the tissue biopsies were negative for any fungal growth. The diagnosis was confirmed based on the positive result of polymerase chain reaction for mucormycosis.

2.6 Treatment of rhino-orbital mucormycosis
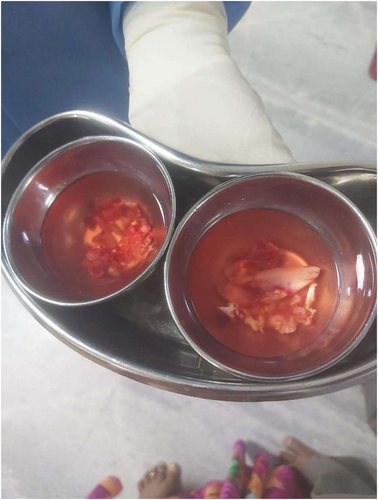
Antifungal treatment was administered as liposomal and lipid amphotericin B at 1–1.5 mg/kg/d doses for 4–6 weeks. It was followed by functional endoscopic sinus surgery and debridement of necrotic and infected tissue on the left side of the face and nose (Figure 4 shows the removal of dead and necrotic tissue after mucormycosis surgery). Treatment was switched to oral posaconazole 300 mg/d during the continuation of ALL maintenance treatment, which continued at home because the spectrum of posaconazole covers mucormycosis and is taken orally. Posaconazole was given for a total of 5 months during the maintenance treatment. The patient remained in remission for ALL and free of fungal infection for a total of 2 years from diagnosis. Subsequent ophthalmological examinations revealed no clinical or radiological signs of active mucormycosis and showed normal conditions of the left eye with no apparent intellectual deficit or reading problems.
2.7 Relapse of B-ALL and its management with hyper-CVAD chemotherapy
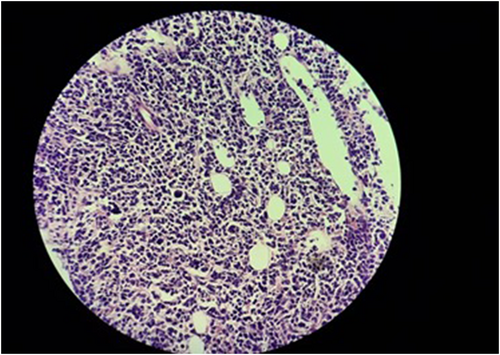
Four months later, the patient presented with complaints of body aches, restlessness, and fever. A relapse of B-ALL was suspected with a peripheral smear showing relative lymphocytosis with left shift and atypical pleomorphic WBCs. A subsequent bone marrow biopsy confirmed the prominence of blasts, accounting for 60% of the mature erythroid and myeloid precursors (Figure 5). The hyper-CVAD chemotherapy regimen consisting of Cycles A and B alternating every 21 days was commenced. Cycle A included cyclophosphamide, dexamethasone, methotrexate, doxorubicin, vincristine, and cytarabine. Cycle B included methotrexate and cytarabine. Each course was given up to four times, with up to eight cycles in total. The regimen was administered on an inpatient basis, using a peripherally inserted central catheter. Following the completion of chemotherapy, blood cell counts returned to normal levels, and hematopoietic stem cell transplantation is currently under consideration.
3 DISCUSSION
Mucormycosis is a rapidly progressing, highly aggressive, and highly fatal opportunistic infection that usually leads to devastating outcomes; thus, it is important to suspect it promptly and treat it as quickly as possible.6 The risk of developing mucormycosis depends on many factors related to the patient's immune status and underlying diseases that may favor the progression and severity of the infection.6, 7
Mucormycosis can be caused by any fungus in the Mucorales order, the most common being the Rhizopus species, followed by the Mucor species.8 The fungus is almost always present in soil, agricultural goods, and both processed and unprocessed food items.9 Immunocompetent individuals eliminate these fungi; however, in those with underlying immunosuppression or ongoing medical conditions, it may result in severe and debilitating disease. Furthermore, several risk factors such as diabetes, malignancy, neutropenia, recurrent diabetic ketoacidosis, iron overload syndromes, corticosteroid use, and bone marrow and solid organ transplantation have been reported to predispose patients to mucormycosis.1, 8
Due to the highly aggressive nature of the disease, the clinical approach to mucormycosis is based on four important aspects: first, suspicion and early diagnosis to promptly initiate therapeutic interventions to prevent progressive tissue invasion and devastating sequelae; second, interruption of the underlying predisposing factors and management of the patient's comorbidities; third, the early administration of active antifungal agents and other types of therapeutic methods; and finally, performing a complete surgical debridement to remove all compromised and infected tissues.7, 10, 11
Furthermore, recent advances in the understanding of mucormycosis include the application of electron microscope. Although, as far as mucormycosis is concerned, from a clinical point of view, electron microscopy has a limited value; it has high experimental significance for pathophysiological understanding. SEM helps us understand the morphology of the nasal sinus microhabitat, sinus dysbiosis, and the morphological bases of the interaction between the microorganism and the cells of the local immune system, highlighting the fact that the way in which the infection develops depends on the interaction of the microorganisms with the cells of the immune system.12, 13 Furthermore, Jeican et al.'s study reported that EM can be a useful tool for the prompt identification of bacterial super-infection.14
In our case, the infection is thought to be induced by the immunosuppressive state as a result of chemotherapy and the underlying B-ALL. Invasive fungal diseases such as invasive aspergillosis and mucormycosis can become life-threatening complications for patients with hematological malignancies as a result of prolonged neutropenia.4 Rhino-orbital mucormycosis in the present case was preceded by a COVID-19 infection. It is believed to contribute to the development of mucormycosis, partly due to the inclusion of steroids in the treatment regimen and a reduction in phagocytic activity following a severe COVID-19 infection.15 Interestingly, our patient only had a mild COVID-19 infection and was not documented to receive steroids.
The mainstay of management is the early deployment of systemic fungal therapy.15 Amphotericin B is the gold standard for antifungal therapy for mucormycosis.15 In addition, surgery is particularly useful in rhino-orbital-cerebral cases and should be performed when necessary, as tissue debridement along with medical therapy is associated with better outcomes than treatment alone.2 Posaconazole, the recommended oral therapy, can be used in addition to or in substitution for amphotericin B therapy. Furthermore, isavuconazole has been approved for adults as the first-line therapy in cases where amphotericin B treatment is unsuitable; albeit, the use of isavuconazole for mucormycosis in children has been reported in the literature.9 Our patient was managed with liposomal and lipid amphotericin B as the first-line antifungal in addition to posaconazole. Medical therapy was followed by surgical excision of the involved areas. The significance of surgery has been demonstrated in several published case series. A single-center study analyzing the impact of combination antifungal therapy for rhino-orbito-cerebral mucormycosis in 41 patients reported that all patients had at least one surgical intervention, showing that the standard approach includes surgical intervention when appropriate.16 In a recent study reviewing 90 patients with rhino-orbito-cerebral mucormycosis and solid organ transplantation, surgical debridement was found to be independently associated with improved outcomes.17
We recommend that clinicians should maintain a high index of suspicion for mucormycosis in immunocompromised patients such as those with hematological malignancies, especially following recent COVID-19 infection and during aggressive chemotherapy regimens. Early diagnosis through imaging and clinical suspicion is crucial for prompt initiation of systemic antifungal therapy and consideration of surgical intervention. A multidisciplinary approach involving infectious disease specialists, oncologists, and surgical teams is paramount for optimal management. Additionally, ongoing education and awareness among healthcare providers regarding risk factors, clinical presentations, and treatment strategies are essential for improving patient outcomes.
The strength of the present study is its documentation of a rare occurrence of rhino-orbital mucormycosis as a result of the immunosuppressive state rendered by B-ALL after COVID-19 infection. Additionally, high-quality CT images are provided that distinctly delineate the characteristics of the disease. The diagnosis of mucormycosis in the present study was confirmed by MRI and a post-contrast study in conjunction with the patient's clinical presentation, as opposed to a definitive tissue diagnosis. The important limitation of this study is that we were unable to find the exact etiological agent on biopsy and histopathological examinations. However, it should be noted that, although, culturing is a reliable method for the identification of specific fungal species, cultures from infected tissue are negative in 50% of the cases with positive microscopy findings. In addition, the different zygomycetes may share similar morphology on histopathology, emphasizing the imperative need to perform molecular identification if culture is negative in mucormycosis suspected patients.6
4 CONCLUSION
As mentioned above, a case report is provided along with a unique co-occurrence of B-ALL and rhino-orbital mucormycosis following a COVID-19 infection. In our patient, relapse of B-ALL is currently under control and hematopoietic stem cell transplantation is under consideration. When clinical indications and risk factors are present physicians should consider rhino-orbital mucormycosis along with other differential diagnoses (including rhinosinusal aspergilloma, Wegner granulomatosis, and pseudomonas lesions), especially in immune-compromised individuals. Patient outcomes can be enhanced by appropriate clinical care, which includes administration of systemic antifungal treatment and quick assessment of alternative diagnosis in the event of therapy failure.
AUTHOR CONTRIBUTIONS
Maneesha Lingarapu: Conceptualization; data curation; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Bisma Shaikh: Project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Ajeet Singh: Project administration; supervision; validation; visualization; writing – original draft; writing – review and editing. Mustafa Ahmed Khan: Validation; visualization; writing – original draft; writing – review and editing. Malik Olatunde Oduoye: Validation; visualization; writing – original draft; writing – review and editing. Nancy Kamboj: Validation; visualization; writing – original draft; writing – review and editing. Mahmood Danishwar: Validation; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We would like to thank the team of clinicians who helped manage this case. We would like to thank the patient and his family members for their cooperation in bringing this case for the betterment of the scientific community.
FUNDING INFORMATION
The authors did not receive any funding for this work.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICS STATEMENT
Ethics approval was not required for this case report.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
GUARANTOR
Malik Olatunde Oduoye.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




