A novel mutation in ANOS1 in a Chinese family with Kallmann syndrome: Case report
Rong Jiang and Xueting Qiu contributed equally to this work.
Key Clinical Message
We reported a novel variant in Kallmann syndrome. It not only determines the clinical importance of whole exome sequencing for identification of genetic pathogenic variants, but also enriches the ANOS1 genetic spectrum of CHH patients in Chinese population.
1 INTRODUCTION
Kallmann syndrome (KS) is a rare genetic heterogeneous disease with multiple inheritance including autosomal dominant, autosomal recessive, and X-linked recessive, classically characterized by the accompanying symptom of hypogonadism and anosmia.1 Approximately two-thirds of the cases present as familial syndromes.2 Over 30 monogenic defects have been described as causes of KS till now,3 including mutations in FGFR1, SOX10, WDR11, PROK2, PROKR2, and, most of all, ANOS1, which was previously named KAL1.4
Here, we present a Chinese boy, the proband of our research, presenting with a clinical diagnosis of KS and a member on his mother's side, carrying an X-linked recessive hemizygous novel mutation. A pathogenic variant was identified in the ANOS1 gene on the X chromosome: c.1187C>A. The variant is a single nucleotide change that introduces a stop codon in exon 8 (p. Ser396Ter). What's more, this mutation has not been previously reported in KS.
2 CASE
The proband in this study was a 10-year-old male referred to the endocrine unit of The Affiliated Suzhou Hospital of Nanjing University Medical School, complaining of delayed puberty and lack of sense of smell. The patient had a past medical history of undescended testicles.
On examination, he showed Tanner developmental stage II, 2 cm penis, and 1 cc testes bilaterally. No beard or pubic hair was revealed. He denied morning erection and ejaculation. Following height (147 cm) and weight (34.8 kg), he showed the body mass index of 16.1, based on the interpretation of anthropometric indices of the World Health Organization.
The patient was born to non-consanguineous parents after a normal pregnancy. At birth, he weighed 3.3 kg and measured 50 cm in crown-heel length. Solitary kidney was founded during treatment of the undescended testicles. No neurologic concerns except anosmia were revealed.
Blood tests found testosterone in the lower end of normal range (0.24 nmol/L total), and low luteinizing hormone (LH) and follicle-stimulating hormone levels (0.09 and 0.37 IU/mL respectively). Laboratory examination demonstrated hypogonadotropic hypogonadism. (Table 1). Baseline T4, thyrotropin and adrenal hormone level were within in the normal range. Chromosomal analysis revealed a 46, XY karyotype.
| Fasting | 30 min later | 60 min later | 90 min later | 120 min later | |
|---|---|---|---|---|---|
| FSH (IU/L) | 0.37 | 1.73 | 1.68 | 2.39 | 3.12 |
| LH (IU/L) | 0.09 | 0.41 | 0.5 | 0.58 | 0.55 |
- Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone.
His mother's 29-year-old cousin showed similar pubertal development. The proband's family pedigree is showed in Figure 1. His blood tests also showed low levels of testosterone and undetectable levels of LH. It is worth noting that solitary kidney can be found on both.
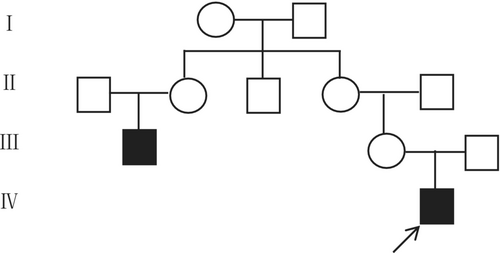
3 METHODS AND RESULTS
Peripheral blood samples were collected from the two patients and their family members (parents and sister). Whole exome sequencing was performed on them. Genetic results were confirmed by Sanger sequencing. The ANOS1 gene of the tested subject exhibits a hemizygous variation of c.1187C>A (p. Ser396Ter) in exon 8. The presence of this hemizygous variant was confirmed in both his sister and mother. The same variant was also found during the cousin uncle's family (Figure 2). However, the other members of the family were not traced for the different regions.
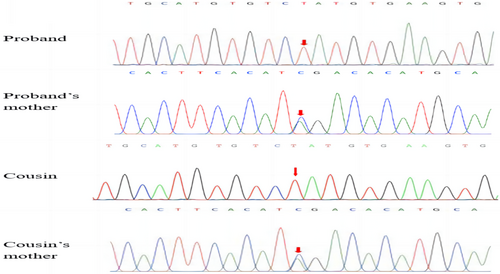
4 DISCUSSION
KS is clinically defined as hypogonadotropic hypogonadism with anosmia. A defect of the migration of gonadotropin-releasing hormone (GnRH) neurons during embryonic development is the main cause, and then leads to a failure of the hypothalamic–hypophysiary axis and a deficit of the subsequent downstream gonadotropins that would otherwise trigger the start of puberty.5 Since KS is a congenital disease, identifying new genetic causes is important for patients to seek appropriate treatment and genetic counseling. However, the genetic cause is identified in only 50% of familial cases and 10% of sporadic ones currently.6
ANOS1 on chromosome X is the gene located in the Xp22.3 region encodes anosmin, which is responsible for the migration of GnRH producing neurons.7 It accounts for 30%–60% of familial and 10%–15% of the sporadic KS cases with known genetic causes.6 Besides KS, some variants of ANOS1 have also been found in disease associated with central nervous system like multiple sclerosis and atopic dermatitis.8
The patients in our study present classic Kallmann phenotype with delayed puberty due to hypogonadal hypogonadism. Significantly, both of them showed solitary kidney, which is less frequent in KS, but common in those with mutation of ANOS1. It adds the proof that ANOS1 plays important role in unilateral renal aplasia. To the best of our knowledge, their variation of c.1187C>A (p. Ser396Ter) has not been reported in ANOS1 before. This transversion mutation in exon 8 encoding tryptophan into a termination codon. Also, Ser396 plays great role in some neuropathological disease like frontotemporal dementia.
5 CONCLUSION
In conclusion, we identified a novel nonsense mutation (c.1187C>A, p. Ser396Ter) of the ANOS1 gene that causes KS. To the best of our knowledge, this is the first genetically confirmed case of KS in the Chinese population.
AUTHOR CONTRIBUTIONS
Rong Jiang: Data curation; writing – original draft; writing – review and editing. Xueting Qiu: Data curation; writing – original draft. Xingfa Han: Writing – original draft. Zhimin Ma: Writing – review and editing.
ACKNOWLEDGMENTS
We thank the patients for their cooperation in this study and all the clinicians for their contribution.
FUNDING INFORMATION
There is no funding in this report.
CONFLICT OF INTEREST STATEMENT
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
ETHICS STATEMENT
Our research was conducted in accordance with the declaration of Helsinki and approved by Clinical Research Ethics Committee of The Affiliated Suzhou Hospital of Nanjing University Medical School.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The authors will supply the relevant data in response to reasonable requests.




