A young woman with Mycobacterium chelonae complex infection caused by facial stem cell injection
Key Clinical Message
In outpatient settings, Mycobacterium chelonae complex infection brought on by cosmetic injections are rather uncommon. We came across a case of infection brought on by a commercial stem cell injection.
1 INTRODUCTION
In the last 30 years, the rise of minimally invasive cosmetic injections has raised patient demand in quicker, more cost-effective treatments. Patients also want faster healing times and a return to normal social activities.1 However, as the use of injectable procedures has increased, there have been reports of significant problems, such as infections, among individuals who have gotten facial injections.1 Lately we've been seeing more cases of atypical mycobacterial infections, mostly in housewives and fishermen who have experienced some kind of trauma, like stabbings. However, it's rare to have it occur due to cosmetic injection, like in this case with a female patient who contracted the Mycobacterium chelonae complex as a result.
2 CASE HISTORY/EXAMINATION
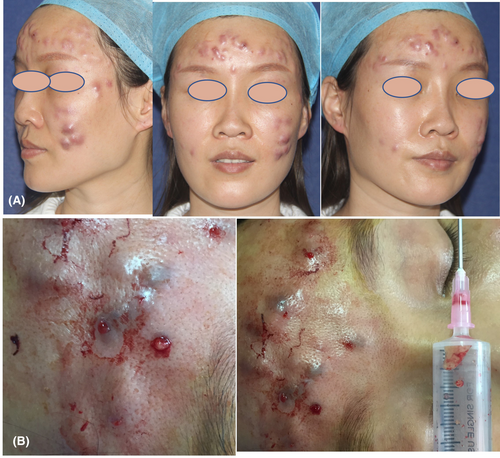
A 33-year-old Asian woman presented with a 14-day history of facial abscesses and nodules (Figure 1A). She had aesthetic treatment with stem cell (non-autologous) injections (including L-glutathione, super oxide dismutase, and bFGF) for 39 days prior to the commencement of the condition in the neighborhood's unofficial beauty parlor. After 5 days of therapy, facial erythema developed; 16 days later, nodules and abscesses did as well. Fourteen days before the presentation, she attended the dermatology clinic in the neighborhood. The dermatologist identified a skin infection and treated it with “clarithromycin” orally and “compound polymyxin B” topically, but nothing changed. Furthermore, a return visit to the hospital 5 days prior and the administration of “Cefdenib” had no effect, and the nodules grew and expanded even more. Because of the short course, the patient was likely to develop skin infection; thus, a skin biopsy was performed, and pustules were extracted for mycobacterial, fungal, and bacterial culture and identification. We gave the patient Levofloxacin 0.5 once a day (qd), Doxycycline 0.1 twice a day (bid), and Rifampicin 0.15 qd in advance before the pathology results because the patient was young and the nodules were growing quickly. We utilized a 20 mL syringe needle to extract pus from larger nodules and abscesses, and the tissues revealed a semi-transparent gel (Figure 1B).
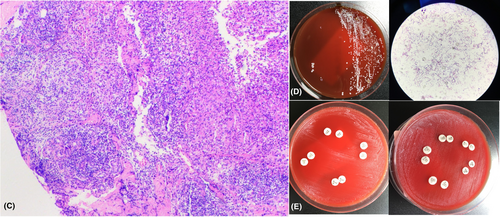
Infiltration of numerous acute and chronic inflammatory cells, the formation of granuloma nodules, and the dispersion of a few multinucleated giant cells were all evident in the pathology of the skin tissue (Figure 2C). Cultures of fungi and bacteria came back empty. Nonetheless, the periodic acid-Schiff staining and mycobacterial culture were both positive (Figure 2D). The amplified 16S rRNA gene was detected as a single, distinct target band. The sequencing results for M. chelonae (GenBank No.042918.1), M. saopaulense (GenBank No.145859.1), and M. abscessus (GenBank No.113128.1) revealed that the sequence similarity with other strains was 100%. Amikacin, Tigecycline, and Ciprofloxacin were shown to be sensitive to drugs while Tetracyline, Imipenem, Cilastatin, Cefoxitin, Linezolid, Ertapenem, and Azithromycin were not (Figure 2E).
3 MDIAGNOSIS AND TREATMENT
M. chelonae was the infection that led to our diagnosis. Given AK (Amicastin) intramuscular injection three alternate day injections (qod) for 2 months, along with clarithromycin 0.25 bid, rifampicin 0.15 qd, and ethambutol 0.25 third a day (tid). We used a 20 mL syringe needle to draw pus from bigger lesions or abscesses.
4 OUTCOME AND FOLLOW-UP
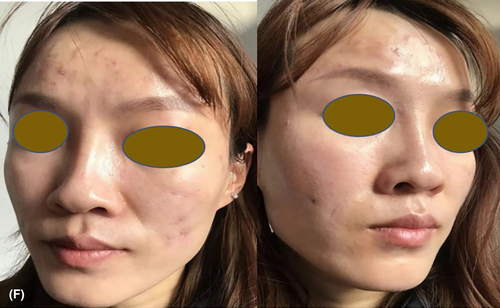
The abscesses and nodules had fully disappeared after 4 months of virtual follow-up, leaving just atrophic scars (Figure 3F).
5 DISCUSSION
Nontuberculous mycobacterium (NTM) is a rapid-growing mycobacteria (RGM).2 M. fortuitum, the M. chelonae/M. abscessus complex, the M. smegmatis group, the M. mucogenicum group, the M. mageritense/M. wolinskyi, and pigmented RGM are the six main groups that make up RGM. The most prevalent clinically relevant species among RGMs are M. fortuitum, M. chelonae, and M. abscessus.3 M. chelonae has been identified in aquatic organisms, water, and soil.4 It frequently results in a broad range of infections, including those that affect the skin and soft tissues, particularly the eyes and extremities.5 Cellulitis, non-healing ulcers, subacute or chronic nodular lesions, abscesses, superficial lymphadenitis, and verrucous lesions are some of the clinical signs of skin infections. We discovered that M.chelonae and M.abscessus exhibit considerably greater susceptibility to aikacin, cefoxitin, and clarithromycin than any other antimicrobial agents2 after reviewing the literature.
The patient quickly developed significant areas of nodules and cysts at the injection site after receiving a cosmetic injection. When the brow wrinkles were injected, a good friend who underwent face surgery alongside her experienced the same issues. Consequently, rather than the potential of a malignant tumor, it should be assumed that this case was caused by infection with certain microbes. We made the assumption that this NTM might have contaminated the injection or unconventional disinfection. Based on skin pathology and the results of gene detection, we identified M. chelonae complex as the cause of the facial illness. The medication was changed for therapy in accordance with the findings of the drug sensitivity test. Concave scars emerged after the skin blemishes disappeared. To use medical cosmetic technology, we think the majority of patients should go to regular hospitals, and the medical personnel should closely regulate cleaning. We anticipate that government agencies will tighten their oversight and administration of aesthetic medical technology.
AUTHOR CONTRIBUTIONS
Kun-jie Li: Resources; writing – original draft. Song-fa Lin: Visualization. Yan-ni Guo: Visualization. Tian-xing xu: Supervision. Chao Ji: Project administration; writing – review and editing.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not- for- profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
CONSENT
Published with the written consent of the patient.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.