Coinfection by Mucoraceae and Aspergillus Species in a Patient With Acute Leukemia: A Clinical Case Report
Funding: The authors received no specific funding for this work.
ABSTRACT
The concurrent occurrence of invasive mold infections caused by two distinct fungal species in hematologic patients represents a rare and challenging condition. The mortality rate associated with these invasive fungal infections in neutropenic patients is high, underscoring the importance of timely diagnosis and prompt initiation of appropriate treatment to improve clinical outcomes. Herein, we present a concomitant acute invasive fungal rhinosinusitis (AIFR) and invasive pulmonary fungal infection caused by Aspergillus and Mucoraceae species in a patient with pre-B-cell acute lymphoblastic leukemia. This case highlights the need for further research to optimize outcomes in immunocompromised patients.
1 Introduction
Hematological malignancies (HMs) are a diverse group of malignant clonal disorders that primarily affect the blood and hematopoietic tissues. These malignancies are broadly categorized into myeloid and lymphoid lineages [1]. Acute lymphoblastic leukemia (ALL) is classified as an acute lymphoid lineage disorder. All HMs compromise the immune system, significantly increasing the risk of opportunistic infections, particularly invasive fungal infections (IFIs) [2].
Aspergillus and Mucorales species have emerged as significant pathogens and are among the most common causes of highly lethal IFIs [3]. Implementing antifungal prophylaxis targeting Candida species in individuals with HMs has led to a shift in fungal epidemiology, resulting in a higher incidence of aspergillosis compared to candidiasis [4].
Aspergillus spp., an airborne fungus, typically manifests in invasive, saprophytic, or allergic forms [5]. Among these, Aspergillus fumigatus (A. fumigatus) is the most frequently implicated species in disease, although other species can also cause invasive infections in profoundly immunocompromised patients [6]. Invasive aspergillosis occurs in 4%–15% of neutropenic patients and carries a mortality rate of 60%–85% [7].
Although less frequent than aspergillosis, Mucormycosis is a devastating angioinvasive fungal infection caused by filamentous fungi of the Mucorales order [8]. It can affect various anatomical sites, including rhino-orbital-cerebral, pulmonary, gastrointestinal, cutaneous, and disseminated forms, with mortality rates ranging from 32% to 70% [9]. While the lung is the most common site of infection in patients with HMs, rhino-orbito-cerebral mucormycosis (ROCM) is predominantly observed in diabetic patients [10].
Severe neutropenia, defined as an absolute neutrophil count below 500 cells/mm3 due to toxic antineoplastic chemotherapy, myelosuppressive agents, or allogeneic hematopoietic stem cell transplantation (allo-HSCT), places patients with HMs at an elevated risk of life-threatening infections that often present with few specific symptoms [11].
2 Case History and Examination
In late November 2023, a 21-year-old man diagnosed with pre-B-cell ALL was admitted to the hematology department of Omid Hospital, affiliated with Isfahan University of Medical Sciences (Isfahan, Iran), to receive induction chemotherapy. The chemotherapy regimen administered was CALGB 10403. Prophylactic medications during chemotherapy included levofloxacin, co-trimoxazole, acyclovir, and fluconazole. Five days after completing induction chemotherapy, the patient developed a fever (T: 38.5°C).
The patient presented with fatigue, occasional dry cough, and left-sided pleuritic chest pain. Vital signs and physical examination were typical, and oxygen saturation was 96% on ambient air.
3 Investigations and Treatment
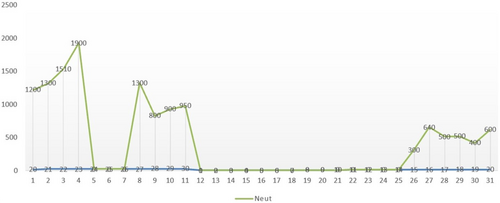
Laboratory testing revealed the following results: white blood cell (WBC) count of 1100/mm3, polymorphonuclear neutrophils (PMN) of 400/mm3, a hemoglobin level of 7 g/dL, platelet (PLT) count of 91,000/mm3, and C-reactive protein (CRP) level of 15 mg/dL. The neutrophil trend during chemotherapy is shown in (Figure 1).
- Meropenem 1 g IV every 8 h
- Ciprofloxacin 400 mg IV every 8 h
- Vancomycin 1 g IV every 12 h
- Liposomal amphotericin B (5 mg/kg) 350 mg IV daily
Nucleic acid amplification tests for influenza A and B and real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) for SARS-CoV-2 were negative. Blood cultures and a serum galactomannan antigen test (using the Platelia Aspergillus ELISA kit, Bio-Rad) were also negative (index value: 0.3).
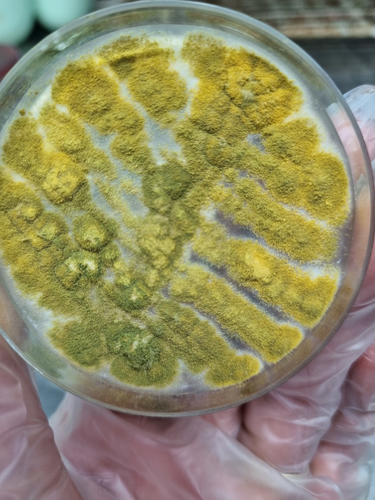
Due to nodular pneumonia and sinusitis, bronchoscopy with bronchoalveolar lavage (BAL) was performed. The BAL sample was analyzed for bacterial and fungal smear, culture, and galactomannan antigen. BAL culture revealed colonies consistent with Mucoraceae species (Figure 4). The BAL galactomannan antigen test returned a high index value of 3.8. Cytopathology testing for malignancy was negative.

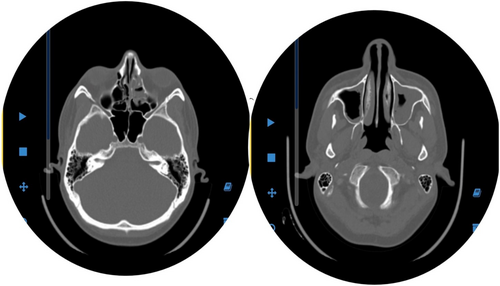
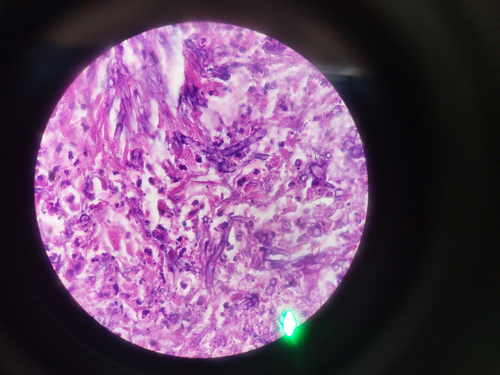
Functional endoscopic sinus surgery (FESS) followed sinus endoscopy findings of scattered pale, necrotic tissue suggestive of invasive fungal sinusitis. Sinus samples were sent for smear, culture, and histopathological evaluation. The culture of the sinus samples revealed colonies consistent with Aspergillus flavus (Figure 5). The histopathological evaluation demonstrated extensive necrosis involving the sinus mucosa and surrounding tissues, with evidence of angioinvasion by broad, aseptate hyphae, consistent with invasive mucormycosis.
Based on these findings, a diagnosis of concomitant probable invasive Aspergillus rhinosinusitis and confirmed invasive Mucor rhinosinusitis, along with probable invasive pulmonary aspergillosis (IPA) and probable pulmonary mucormycosis, was established.
4 Outcome and Follow-Up
After 7 days of therapy, a second-look sinus endoscopy revealed no evidence of necrosis. Meropenem, ciprofloxacin, and vancomycin were discontinued, and treatment with liposomal amphotericin B was continued. The patient became afebrile by Day 5 of treatment.
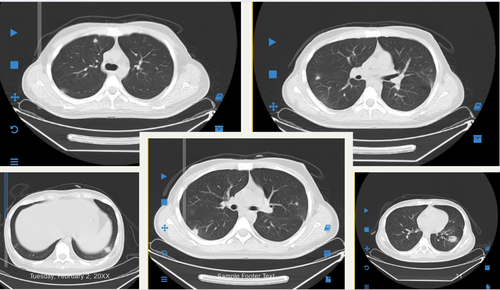
A repeated chest HRCT performed after 22 days of antifungal therapy showed a slight reduction in the size and number of the nodular lesions. To facilitate the start of consolidation chemotherapy, a high-dose methotrexate and cytarabine (ara-C) regimen with leucovorin rescue was initiated 22 days after the start of liposomal amphotericin B therapy.
Throughout four ENT consultations, no evidence of necrosis was observed during repeated sinus endoscopies. A follow-up chest HRCT revealed significant improvement in the pulmonary nodular lesions. After 37 days of therapy, liposomal amphotericin B was overlapped with posaconazole suspension (200 mg every 6 h for 5 days), after which liposomal amphotericin B was discontinued. Posaconazole suspension was continued at a therapeutic dose for 30 days (Figure 6).
The patient had been followed for 5 months with no evidence of infection recurrence. Chemotherapy courses are ongoing under posaconazole secondary prophylaxis. The treatment timeline is illustrated in (Figure 7).

5 Discussion
Simultaneous dual-invasive mold infections are sporadic and life-threatening in immunocompromised patients, posing significant diagnostic and therapeutic challenges [12]. This report presents a unique case of concurrent sino-pulmonary aspergillosis and mucormycosis in a 21-year-old patient with pre-B-cell ALL, where severe and prolonged neutropenia induced by chemotherapy was identified as a significant predisposing factor.
Early and accurate diagnosis is critical for improving outcomes in high-risk patients. A multidisciplinary approach incorporating radiological, histological, microbiological, and serological findings is pivotal [13]. HRCT, with its high sensitivity, is essential for detecting fungal pneumonia and assessing sinus involvement. In neutropenic patients with IPA, characteristic HRCT features include nodules or opacities with a “halo sign” [14]. CT imaging also effectively delineates the extent of fungal sinus lesions, signifying fungal sinus infection [15].
When cultures are negative, histopathology remains crucial for identifying fungal hyphae. Tissue biopsy, combined with molecular techniques such as PCR or immunohistochemistry, enhances diagnostic accuracy by identifying fungal species and detecting drug resistance [16]. PCR assays detect fungal DNA in blood, serum, plasma, or BAL samples and are instrumental in monitoring treatment response, as treatment-induced PCR negativity correlates with improved survival [17]. Additionally, detecting Aspergillus galactomannan (GM) antigen in BAL fluid supports a diagnosis of probable IPA, as observed in this case [18].
Based on the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria, invasive fungal infections are classified as probable or confirmed according to clinical, radiological, and microbiological criteria [19]. In this case, probable IPA and Aspergillus invasive fungal rhinosinusitis (AIFR) were diagnosed based on positive BAL and sinus cultures. Concurrently, confirmed mucormycosis was established through histopathological findings of broad, aseptate hyphae, and probable pulmonary mucormycosis was suggested by positive BAL cultures for Mucoraceae species [19]. The European Confederation of Medical Mycology (ECMM) and European Conference on Infections in Leukemia (ECIL) guidelines recommend mold-active antifungal therapy combined with surgical intervention and control of underlying conditions. Voriconazole is the first-line agent for IPA, while liposomal amphotericin B (L-AmB) is the treatment of choice for mucormycosis due to its broad antifungal activity and reduced nephrotoxicity compared to other formulations [20, 21]. In previous reports, high-dose L-AmB has also been shown to successfully treat coinfections caused by Aspergillus and Mucoraceae species [22]. Patients receiving higher-dose regimens of L-AmB tend to achieve better cure rates, albeit at the cost of more frequent renal adverse effects [23, 24]. Its advantages over other amphotericin B formulations, including lower nephrotoxicity and fewer infusion-related reactions (e.g., fever and rigors), make it the preferred option in high-risk cases [23].
Combination antifungal therapy [24] is not recommended as a first-line treatment due to its lack of significant reduction in mortality rates. It is reserved for refractory cases or patients unable to tolerate high-dose L-AmB due to adverse effects [25]. Even though treatment options remain limited for patients with hematologic malignancies, early diagnosis and prompt treatment allowed this patient to survive.
This report underscores the rarity and clinical significance of dual invasive fungal infections, highlighting the importance of rapid diagnosis through a combination of radiological, histological, and molecular techniques. When feasible, early antifungal therapy and surgical intervention are critical for improving outcomes. Importantly, this case provides new insights into managing dual invasive fungal infections by demonstrating the effectiveness of monotherapy with L-AmB, supporting its role in treating similarly complex cases.
Given the limited literature and lack of standardized treatment protocols, further research is needed to establish evidence-based guidelines for managing these challenging infections. Comprehensive studies focusing on optimal diagnostic tools and therapeutic strategies will be essential for advancing clinical management and improving survival rates in patients with hematologic malignancies and profound immunosuppression.
In conclusion, this case report underscores the importance of recognizing concurrent sino-pulmonary aspergillosis and mucormycosis coinfection in a patient with acute leukemia, a condition rarely documented in the literature.
Optimizing the management of such coinfections necessitates a high index of suspicion, early detection of clinical signs and symptoms through a multidisciplinary approach, targeted antifungal therapy tailored to pathogen sensitivity and patient factors (e.g., renal function, drug interactions), and, when necessary, aggressive surgical resection to reduce fungal burden.
Given the lack of consensus on treating mixed IFIs, a more potent and targeted therapeutic approach may be necessary than the treatments for infections caused by a single fungal agent. Further studies are needed to establish evidence-based guidelines for managing dual-IFIs.
Author Contributions
Atousa Hakamifard: conceptualization, writing – review and editing. Mohammadsaleh Peikar: conceptualization, data curation. Seyed Amirhossein Dormiani Tabatabaei: data curation, writing – original draft.
Ethics Statement
This study was approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.ARI.MUI.REC.1403.094).
Consent
Written informed consent was obtained from the patient for publication of the de-identified data in this case report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




